- LOGIN
- MemberShip
- 2025-12-22 08:07:45
- Company
- 'Adempas' for pulmonary hypertension closer to obtain reimb
- by Eo, Yun-Ho May 14, 2025 06:11am
- Product photo of Adempas A new reimbursable treatment option for pulmonary hypertension is anticipated to be introduced. According to industry sources, Bayer Korea has finally reached an agreement with the National Health Insurance Service (NHIS) for its 'Adempas (riociguat).' Accordingly, after 10 years since it was approved in South Korea, Adempas will likely be included in the reimbursement list. Adempas' negotiation was a dosage negotiation, rather than a ceiling price negotiation. Bayer accepted the price 100% below the weighted average price (WAP) of a substitute drug and passed the Drug Reimbursement Evaluation Committee (DREC) of the Health Insurance Review and Assessment Service (HIRA) in February. Adempas was exempted from the drug pricing negotiations. This drug obtained approval in South Korea as an orphan drug in June 2014. Five products with different doses are available, and it has the efficacy and the effect in ▲patients with persistent/recurrent chronic-thromboembolic pulmonary hypertension (CTEPH, WHO Group 4) after surgical treatment or inoperable CTEPH, to improve exercise capacity ▲adult patients with arterial pulmonary hypertension (PAH, WHO Group 1) who have WHO functional class 2-3, to improve exercise capacity. Adempas has been known as the first novel drug to treat CTEPH. CTEPH occurs in patients with chronic pulmonary embolism who progress to chronic obstructive pulmonary disease (COPD) and develop fibrotic stenosis and occlusion, leading to pathological vascular remodeling and increased resistance in the pulmonary arteries. CTEPH is a chronic disease that causes progressive shortness of breath and right heart failure. Symptoms include dyspnea, fatigue, chest pain, dizziness, peripheral edema, cough, and hemoptysis, significantly impacting quality of life. Ultimately, it can progress to heart, kidney, and liver failure, potentially leading to death. Meanwhile, Adempas is a stimulator of soluble guanylate cyclase (sGC), an enzyme found in cardiovascular organs. The efficacy of the drug in patients with chronic thromboembolic pulmonary hypertension (CTEPH) was confirmed in Phase 2 and Phase 3 clinical trials. The clinical trial results showed that Adempas improved the study's primary endpoint physical activity and demonstrated superior tolerability. No unusual adverse reactions were reported. In the CHEST-1 study, results from comparing the 6 Minute Walking Test (6MWT) at 16 weeks from baseline showed that the patient group treated with riociguat had statistically significant improvement compared to the placebo group. In the PATENT-1 study, comparison of changes in 6MWT values at 12 weeks to the placebo group demonstrated statistically significant improvement, meeting the primary endpoint.
- Company
- U.S. executive order on drug price cuts raises hope
- by Kim, Jin-Gu May 14, 2025 06:10am
- U.S. President Donald Trump has signed an executive order to significantly reduce drug prices in the United States. This measure, which focuses on significantly reducing U.S. drug prices in line with overseas prices, is expected to have a positive effect on domestic biosimilars. However, some predict that the expected effects of this executive order will not be sufficient and that there will be no significant impact. Trump signs executive order to lower drug prices in the US... “Up to 90% reduction” According to local media reports on the 13th, President Trump signed an executive order on the 12th (local time) to lower the prices of prescription drugs in the US to the same level as other countries. The order essentially applies the most-favored-nation policy to drug prices in the US. Under the executive order, the US Secretary of Health and Human Services must promote a program that allows US patients to purchase drugs directly from pharmaceutical companies at a “most-favored-nation” price. In addition, the Secretary of Health and Human Services must impose the most-favored-nation pricing on companies within the US pharmaceutical industry within 30 days. This measure is interpreted as a move made to present a kind of price cap to pharmaceutical companies compared to drug prices overseas, and pressure them to lower their prices accordingly. Ultimately, this is expected to improve the intermediate distribution structure of Pharmacy Benefit Managers(PBMs) and further reduce the prices of expensive drugs. President Trump said at a press conference, “What we are trying to do is level the playing field for drug prices,” adding, “The American people will be able to purchase drugs at the lowest prices in the world.” He did not specify which drugs would be subject to price reductions. However, the administration has hinted that the price reduction could be as high as 90%. President Trump said at the press conference, “Drug prices in the United States could be reduced by 59%, 80%, or even 90%.” A day earlier, he had posted on his Truth Social account, “Prescription Drug and Pharmaceutical prices will be REDUCED, almost immediately, by 30% to 80%.” “Original drug price cuts expected to expand market for Korean biosimilars” The domestic pharmaceutical and biotech industry has responded with more optimism than concern to these measures. There are expectations that the price cuts targeting original drugs will have a positive impact on biosimilars. Celltrion said it expects the measure to create “a better business environment.” With the simplification of the intermediate distribution structure, it is expected that “this will provide positive opportunities for Celltrion's U.S. business activities,” and that “the dominance of pharmaceutical companies making high profit selling original products is expected to weaken, which could present market expansion opportunities for biosimilar companies.” Furthermore, it is anticipated that “biosimilar manufacturers will be able to negotiate drug prices directly with the government rather than through intermediaries such as PBMs, which could benefit both the government and manufacturers.” Additionally, it predicted that the prescription of biosimilars will increase as the prices of high-priced drugs are reduced. Currently in the US, through insurance companies and PBM systems, high-priced original drugs are prioritized for inclusion in formularies, followed by limited competition among biosimilar products, resulting in the addition of 2–3 products. During this process, rebates are paid to intermediary distributors, so the burden associated with these rebates will be significantly reduced with the new system. Celltrion stated, “Previously, biosimilar prices were set at the same high level as originals, making it impossible to provide substantial benefits to patients. However, if the intermediate distribution structure is improved through this executive order, the actual prescription prices of biosimilars will decrease, ultimately expanding biosimilar prescriptions to European levels.” Furthermore, it is also expected to provide an opportunity to launch new products in the US market. Celltrion explained, “If parallel imports are activated to supply medicines at most-favored-nation prices in accordance with this executive order, Celltrion will secure the opportunity to launch additional products that have not yet been introduced in the US market.” Another biosimilar company, Samsung Bioepis, is said to have shown a similar response. A positive outlook on the newly announced measure is dominant both inside and outside the company. The background is the expectation that the preferential policy for biosimilars will be strengthened to reduce medical costs. However, a Samsung Bioepis official said, “We are closely monitoring this policy,” without further elaboration. “It is premature to make specific judgements… Its impact on the Korean pharmaceutical industry will be limited The key issue is the strong opposition from the U.S. pharmaceutical and biotech industry. The U.S. pharmaceutical and biotech industry has consistently opposed the U.S. government's repeated attempts to lower drug prices, either by blocking them altogether or minimizing their impact. As a result, the actual evaluated effect of the drug price reductions was then minimal. In fact, President Trump signed an executive order for drug price cuts during his first term in 2018, but it was ultimately scrapped due to opposition from the pharmaceutical industry. At the time, an attempt was made to lower drug prices through an international reference pricing system, but a federal court raised procedural issues and put the brakes on the plan. President Joe Biden also conducted drug price negotiations with major pharmaceutical companies under the Inflation Reduction Act (IRA). However, the actual drug price reductions were limited to 10 drugs, including Eliquis, Xarelto, Januvia, Forxiga, Entresto, Enbrel, Imbruvica, Stelara, and Fiasp. Even these are limited to those who have Medicare - people aged 65 and older - in the United States. The drug price reduction measures will take effect in 2026, and the annual savings in medical costs are estimated at USD 6 billion (approximately KRW 8 trillion). This is considered insignificant compared to the total drug costs in the United States, which amounted to USD 805.9 billion (approximately KRW 1,142 trillion) last year. In this situation, the Trump administration's push for more stringent drug price reduction policies in the second term is expected to face strong opposition from the U.S. pharmaceutical industry. Additionally, criticism has emerged locally that the executive order lacks specific policies. The New York Times (NYT) criticized the executive order, stating that it “did not include specific policies such as pushing for legislation to reduce drug prices or revising drug payment regulations under government health programs.” In the same line, there are also projections that its impact on the domestic pharmaceutical and biotech industry will be limited. A representative from the pharmaceutical industry stated, “So far, only the direction to pursue drug price cuts has been outlined; there are no specific methods for how the cuts will be implemented, nor have the target products been determined.” The official added, “Its impact on domestic pharmaceutical and biotech companies is currently unclear, and even if there is an impact, it is expected to be minimal.” Another industry insider noted, “The recent administrative order requiring the manufacture of pharmaceuticals within the United States, as well as the upcoming pharmaceutical tariff policy expected next week, are likely to have a greater impact on the domestic pharmaceutical and biotech industry than the recent drug price reduction administrative order.”
- Product
- Patient organization proposes 6 policies as election pledges
- by Kang, Hye-Kyung May 14, 2025 06:10am
- A patient group has proposed six policies, including a “rapid reimbursement for new drugs and post-marketing adjustment system.” The Korea Alliance of Patients Organization submitted a 'Statement regarding Six Major Patient Policies' to political parties on the first day of the official campaign for the 21st presidential election on the 12th, urging them to reflect the proposals in their election pledges. The six proposed policies are: ▲Enactment of a Framework Act for patients to promote their treatment journey and protect their rights ▲Establishment of a Patient Policy Division within the Ministry of Health and Welfare ▲Establishment of an integrated support platform for patients fighting illness ▲Introduction of a system for rapid reimbursement and post-marketing adjustments for new drugs directly related to life ▲Institutionalization of a nursing care system ▲Promotion of a national responsibility system for essential organ transplant costs. KAPO stated, “The medical crisis that has lasted over a year and three months, triggered by the government's policy to increase medical school quotas. The resulting conflict with the medical community has caused immense suffering and harm to us patients. The conclusion of this medical-political conflict has left only a visual lesson: the government cannot defeat doctors by using patients' lives as a tool. Now, we patients and patient groups demand a government that will protect patients' lives and rights no matter what medical crisis arises.” They called for a government that will not hesitate to push forward policies and legislation for patients' treatment and rights even in the face of opposition from the medical community, and for a presidential candidate who will create a “patient-centered healthcare environment” where patients are no longer objects or targets but as “subjects” that actively participate for their treatment and rights. They stated, “We have conveyed patients' voices to presidential candidates as 6 major patient policies and hope they will be adopted as campaign pledges.” KAPO was established on February 4, 2010, under the slogan “Listen to Patients,” and currently includes the Korean Leukemia Patients Organization, the Korean GIST Patients Association, the Korea Kidney Cancer Association, All. Can Korea, the Korea Congential Heart Disease Patient Group, the Korea Psoriasis Association, the Korean Society of Type 1 Diabetes, the Korea Neuroendocrine Tumor Society, the Korean PROS Patient Organization, and the Korean Parkinson's Hope Association, among others, with over 92,000 patient participants.
- Company
- How has Leqembi changed the clinical site in 6 months?
- by Moon, sung-ho May 14, 2025 06:09am
- The overall treatment system on-site has been changing with the introduction of Leqembi, a new dementia drug that was released 6 months ago in Korea. In response, experts are evaluating the therapeutic effects and side effects of Leqembi (lecanemab, Eisai Korea) while demanding improvements to the health insurance reimbursement system in various areas, such as testing costs. At the same time, they are expressing anticipation for other new dementia drugs from global pharmaceutical companies that are likely to be introduced in Korea. The introduction of just one new global drug has changed the atmosphere on-site and in academic activities. #According to the medical community on the 12th, Eisai Korea’s Leqembi (Lecanemab), which was launched in Korea at the end of last year, has passed the Drug Committees (DCs) of major university hospitals and is rapidly expanding its prescriptions in Korea. Leqembi is a new treatment that removes amyloid beta (Aβ), one of the main causative substances of Alzheimer's disease. It specifically binds to soluble amyloid beta protofibrils and insoluble amyloid beta fibrils—the most toxic forms of amyloid beta—to reduce amyloid beta plaques in the brain. In particular, it is the first antibody therapy to receive full approval from the FDA in July 2023 for its efficacy and safety in delaying the progression of Alzheimer's disease and cognitive decline by removing the causative agent. In Korea, it was approved by the MFDS in May last year and has been prescribed in general hospitals since the end of November. However, there are limitations in that it can only be used in medical institutions that meet certain standards, as it requires collaboration with radiologists, neurologists, or other specialists to assess amyloid-related imaging abnormalities (ARIA) such as cerebral vascular lesions and cerebral hemorrhage, as well as facilities capable of administering Leqembi intravenously every 2 weeks and personnel to monitor adverse drug reactions. Nevertheless, Lecanemab is being rapidly adopted and utilized on-site not only at university hospitals but also at regional hub general hospitals, due to the disease's characteristic lack of a fundamental cure. According to an internal evaluation by the Korean Dementia Association, approximately 700 cases have been treated using the medication 6 months after its introduction in Korea. Seong Hye Choi, president of the Korean Dementia Association (Department of Neurology, Inha University Hospital), stated, “A patient weighing 50kg bears approximately KRW 1 million for a single administration of Leqembi. Considering that it is administered every 2 weeks, this amounts to KRW 2 million per month, and KRW 24 million per year for the patient. For a 40kg patient, the cost per administration is approximately KRW 800,000. Some patients are covered with indemnity insurance, and the treatment being well received onsite, even better than expectations.” Additionally, in clinical settings, there is a projection that the incidence of adverse reactions related to brain edema, particularly ARIA, observed during MRI scans in the course of Leqembi’s introduction may be relatively lower than what was shown in clinical studies. Kee Hyung Park, Chair of the Strategy and Planning Committee (Department of Neurology, Gachon University Gil Medical Center), said, “Japan introduced Leqembi before South Korea, and the drug is being actively used as it is covered by reimbursement. Among approximately 8,000 cases, ARIA occurred in 537 cases, which is 6.7% of all patients.” He added, “During the initial clinical trials, the incidence rate of ARIA was reported to be between 12% and 17%.” Park added, “In Korea, there have been no cases of patients experiencing severe side effects after receiving Leqembi. While it is still too early to draw definitive conclusions, it is reasonable to expect that side effects may be less common in Asians compared to Westerners. Amidst this, doctors have suggested that reimbursement for Leqembi itself is necessary, but that reimbursement for tests such as MRI is more urgent. Currently, MRI tests are required to assess cerebral vascular lesions and ARIA in patients receiving Leqembi. The logic is that tests that bring less health insurance burden should be reimbursed first, followed by a discussion on reimbursement for the drug itself. Choi said, “According to MFDS guidelines, patients receiving Leqembi must undergo MRI scans. This means that MRI scans must be performed before the fifth, seventh, and fourteenth injections. If reimbursement is applied to this part of the treatment, it will be able to somewhat reduce the burden on patients.” Choi also pointed out that long-term support for the costs of injection rooms and dedicated nurses (coordinators) provided for severe cancer patients is also necessary for dementia. Recently, Ewha Womans University Mokdong Hospital and Ewha Womans University Seoul Hospital introduced dedicated nurses following the adoption of Leqembi, but many medical institutions find it difficult to operate such programs due to the burden of labor costs. Jee Hyang Jeong of the Department of Neurology at Ewha Womans University Seoul Hospital (Chair, Public Relations Committee, KDA) explained, “When patients and their families request Leqembi treatment, it is necessary to provide detailed explanations about the treatment process, side effects, MRI scans, and other related matters. This entire process typically takes 30 to 40 minutes. We recognize this as a critically important process and have begun operating with dedicated staff internally. This was a big and dedicated decision on the hospital’s part.” Jeong added, “Ultimately, dementia is similar to cancer. We need to hire dementia specialists to educate patients and their families. The same applies to infusion rooms. Comprehensive improvements are needed, including reimbursement for the use of infusion rooms and labor costs, similar to cancer treatment.” Following the use of Leqembi on-site, expectations are also rising for another new dementia drug, Lilly's Kisunla (donanemab). Lilly's Kisunla cleared the regulatory hurdle - FDA - last year. Kisunla demonstrated efficacy in delaying cognitive decline in patients with early-stage Alzheimer's disease in the Phase III TRAILBLAZER-ALZ2 study. In the clinical trial, it delayed cognitive decline regardless of the disease's progression or pathological stage. Particularly, compared to Leqembi, it is evaluated as having a high potential for utilization in Korea due to improved convenience in patient administration. Choi said, “Kisunla has the advantage of a relatively long administration cycle and the possibility of discontinuing treatment midway through the course. I understand that Lilly, the manufacturer of Kisunla, is in discussions with the MFDS for its introduction in Korea. With mixed results in global markets, I expect the MFDS to make a decision based on the domestic situation.” Park also noted, “Rather than focusing on why approval was granted in the U.S. but not in Europe, we should examine the characteristics of the drug and its differences from existing medications. We need to assess this and consider how patients can individually select the appropriate medication for treatment when approval is granted domestically.”
- Policy
- Reimb of comb cancer therapies w/o detailed criteria
- by Lee, Tak-Sun May 14, 2025 06:09am
- The reimbursement policy for combination cancer therapies, announced this month, has been implemented without detailed criteria, causing further confusion in medical practices. This was contributed by the Ministry of Health and Welfare (MOHW) and the Health Insurance Review and Assessment Service (HIRA), which had differing schedules for revision. Accordingly, the HIRA will convene a Cancer Drug Review Committee (CDRC) meeting, notify of detailed criteria, and implement it in June. As of May 1, the MOHW approved that 'when combining a reimbursed chemotherapy regimen with another anticancer drug, the existing co-payment for the previously initiated chemotherapy shall continue to apply to that regimen.' Previously, all combination therapies not approved by insurance reimbursement were entirely non-reimbursed. However, with the establishment of this clause, one anticancer agent now recognized for reimbursement is expected to alleviate patients' financial burden and improve treatment accessibility. Patient organizations and foreign pharmaceutical companies have welcomed such notification. The problem is that no detailed criteria for reimbursement eligibility have been issued, confusing the field. An industry professional explained, "Even hospitals don't know whether the combination therapy will be covered when prescribing it, so they ask the sales representatives, but the pharmaceutical companies themselves cannot provide a clear answer." There have also been repeated inquiries about which of the two drugs in an approved combination regimen is eligible for reimbursement. The MOHW's revision containing the principles for partial reimbursement of combination cancer therapies (Detailed Criteria and Methods for Applying Reimbursement-Drugs) was announced on April 28. It took effect on May 1. Meanwhile, the CDRC, which was to discuss the HIRA's detailed notice on 'Detailed Criteria and Methods for Applying Reimbursement (of Medications Prescribed·Administered to Cancer Patients),' convened on April 30. In other words, the CDRC met to discuss the details just two days after the notice was announced, and reportedly did not have enough time for proper deliberation. In its administrative notice explaining the reasons for the revision, the MOHW stated that, since the dosing criteria for agents used in anticancer regimens are scheduled to change in the 'Detailed Criteria and Methods for Applying Reimbursement (of Medications Prescribed·Administered to Cancer Patients),' (the HIRA announcement), it would simultaneously revise the cost-sharing regulations; however, it pushed the notice through before HIRA's detailed announcement was issued. This suggests that the MOHW and HIRA did not adequately coordinate on the timing of implementation. It is reported that the two sides had differing views on preparing the list of partially reimbursable drugs. Critics argue that the MOHW rushed a policy that will require massive additional spending. Because setting reimbursement criteria for anticancer drugs falls under both HIRA and the MOHW, issuing the notice without consultation with the NHIS, which administers reimbursements, has been controversial. Some suggest that the MOHW bowed to pressure from the National Assembly, patient groups, and pharmaceutical companies without adequately debating fiscal savings. Notably, a policy forum on improving patient access to combination therapies was hosted by Rep. Lee Joo-young's office in March, during which government representatives expressed caution. Yet, barely two months later, the notice was issued. An unintended side effect of partial reimbursement for combination therapies is that if new drugs are used in first-line treatment, there may be no reimbursable options for second-line therapy after failure. To reduce confusion and unintended outcomes, HIRA plans to convene an unscheduled CDRC meeting this month to finalize the details. In a letter sent to relevant organizations on the 7th, HIRA stated that it will "discuss as soon as possible at the CDRC meeting the approved indications and academic society opinions to reduce confusion in practices when applying the detailed notice to existing anticancer regimens and combination therapies," and that it intends to implement the 'Detailed Criteria and Methods for Applying Reimbursement (of Medications Prescribed·Administered to Cancer Patients)' on June 1.
- Company
- Vyloy quickly lands in general hospitals in Korea
- by Eo, Yun-Ho May 13, 2025 06:07am
- Vyloy, a targeted anticancer drug for gastric cancer, may be prescribed at general hospitals in Korea. According to industry sources, Vyloy (zolbetuximab), a Claudin 18.2-positive gastric cancer targeted therapy developed by Astellas Korea, is now available for prescription at the Big 5 tertiary hospitals in Korea, including Samsung Medical Center, Seoul National University Hospital, Seoul St. Mary's Hospital, Asan Medcial Center, and Sinchon Severance Hospital. The drug has passed the regular or emergency drug committees (DCs) at each hospital. Additionally, prescription codes have been generated at other medical institutions such as Ajou University Hospital, Chilgok Kyungpook National University Hospital, and Hwasun Chonnam National University Hospital. Approved in Korea last September, Vyloy is the world's first claudin 18.2-targeted therapy, an immunoglobulin (IgG) monoclonal antibody that binds to Claudin 18.2, a protein expressed on the surface of cancer cells in gastric epithelial cells. The SPOTLIGHT study, which became the basis of Vyloy’s approval, showed that Vyloy+ mFOLFOX6 (fluorouracil, leucovorin, oxaliplatin) combination therapy’s progression-free survival (PFS) was 10.6 months, compared with the 8.67 months in the placebo arm. Also, the median overall survival (OS) was 18.23 months, compared with the 15.54 months in the placebo arm. Additionally, in the GLOW study, the combination therapy group of Vyloy and CAPOX (capecitabine and oxaliplatin) achieved a median progression-free survival (mPFS) of 8.21 months, reducing the risk of disease progression or death by approximately 31%. However, Vyloy has not yet been reimbursed by health insurance. The drug was submitted to the Health Insurance Review and Assessment Service's Cancer Disease Review Committee in February, but failed to set the reimbursement criteria. Additionally, due to companion diagnostic issues last year, Vyloy was only officially launched in Korea in March. To use Vyloy, patients must be diagnosed as Claudin 18.2 positive, and the companion diagnostic (CDx) device used for Claudin 18.2 diagnosis was considered for evaluation as a new health technology in the reimbursement review process. In response, Astellas has been conducting an EAP program even before Vyloy’s approval to allow sooner access to the treatment, and currently, 51 patients are enrolled across 10 institutions. SunYoung Rha, a professor of medical oncology at Yonsei Cancer Center, stated, “Approximately 90% of metastatic gastric cancer patients are HER2-negative, rendering a dire need for treatment options targeting this biomarker. Given that about 40% of HER2-negative patients are reported to be Claudin 18.2-positive, the release of Vyloy, which selectively binds to Claudin 18.2, presents a new treatment possibility.”
- Company
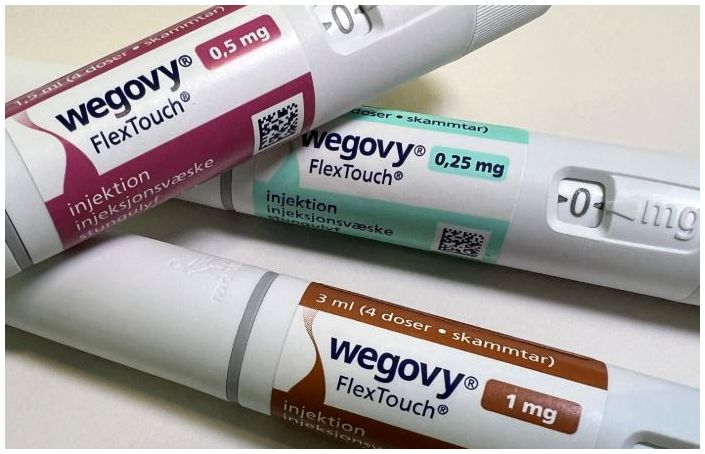
- Mounjaro provides more weight loss than Wegovy
- by Eo, Yun-Ho May 13, 2025 06:06am
- Research results found Mounjaro brings more weight loss than its competitor Wegovy. On the 12th, Lilly announced the detailed results of the SURMOUNT-5 Phase IIIb open-label clinical trial directly comparing Mounjaro (tirzepatide), a dual GIP/GLP-1 receptor agonist, and Wegovy (semaglutide), a GLP-1 receptor agonist. The study, which compared the weight loss effects of the two drugs that have garnered global attention as obesity treatments, was simultaneously presented at the 32nd European Congress on Obesity (ECO) and published in the international academic journal The New England Journal of Medicine (NEJM). The study enrolled adult patients with a body mass index (BMI) of 30 kg/m² or higher, or overweight patients (BMI 27 kg/m² or higher but less than 30 kg/m²) with at least one weight-related comorbidity, excluding diabetes. In the trial, Mounjaro demonstrated superiority over Wegovy, meeting all the primary endpoints and five key secondary endpoints at week 72. The primary endpoint, the mean percent change in weight from baseline to week 72 in the Mounjaro group (10 mg or 15 mg), was 20.2%, compared to 13.7% in the Wegovy group (1.7 mg or 2.4 mg), representing a 47% relative weight loss improvement. Based on treatment regimen estimands, the mean weight loss was 22.8 kg in the Mounjaro group and 15.0 kg in the semaglutide group. Mounjaro also outperformed Wegovy in all key secondary endpoints of weight loss. In key secondary endpoints, Mounjaro was superior across all weight reduction targets, with 64.6% of patients in the Mounjaro group achieving at least 15.0% weight loss compared to 40.1% in the Wegovy group. Additionally, patients in the Mounjaro group achieved a superior average waist circumference reduction of 7.2 in (18.4 cm), while in the Wegovy group saw an average reduction of 5.1 in (13.0 cm). Min-Seon Kim, President of the Korean Society for the Study of Obesity and Professor of Endocrinology at Asan Medical Center, stated, “In the head-to-head trial (SURMOUNT-5), Mounjaro demonstrated a superior weight loss effect compared to Wegovy. I expect the introduction of this medication to contribute to obesity treatment in Korea.”
- Policy
- CDRC to talk partial reimb details for comb cancer therapy
- by Lee, Tak-Sun May 13, 2025 06:06am
- An additional date has been set to discuss detailed criteria regarding partial reimbursement of combination cancer therapy, as announced this month. The Health Insurance Review and Assessment Service (HIRA) has decided to convene another Cancer Drug Review Committee (CDRC) meeting, which was not scheduled. According to industry sources on May 12, the HIRA will convene the 4th CDRC of 2025 on May 14, 4PM at Kukje Electronics Center. During the meeting, detailed criteria related to partial reimbursement of combination cancer therapy, notified by the Ministry of Health and Welfare (MOHW) this month, will be discussed. Based on the discussion during the meeting, the HIRA will revise the 'Detailed Criteria and Methods for Applying Reimbursement (of Medications Prescribed·Administered to Cancer Patients),' then implement it from June. The upcoming CDRC meeting had not been scheduled. The 4th CDRC meeting was initially planned to convene on June 11, but it has been moved up a month. In other words, it is the major issue in medical practices. This month, the Ministry of Health and Welfare (MOHW) newly established a partial reimbursement of combination cancer therapy through partial revision to the 'Detailed Criteria and Methods for Applying Reimbursement (Drugs).' In detail, a new clause states, 'when combining a reimbursed chemotherapy regimen with another anticancer drug, the existing co-payment for the previously initiated chemotherapy shall continue to apply to that regimen.' Previously, all combination therapies not approved by insurance reimbursement were entirely non-reimbursed. However, with the establishment of this clause, one anticancer agent now recognized for reimbursement is expected to alleviate patients' financial burden and improve treatment accessibility. However, confusion persists in medical practices due to a lack of details, such as which drugs are eligible for reimbursement. Pharmaceutical companies have also been asked whether the drug in question is eligible for reimbursement, but they have also been unable to provide clear answers. At the CDRC meeting held on April 30, there was consensus on the need for detailed criteria. Still, they failed to prepare a revision with only a few days left before the MOHW's notification takes effect. Recently, the HIRA sent an official letter to relevant organizations stating that it would promptly discuss at the CDRC meeting the comprehensive consideration of the approved indications and academic society opinions to reduce confusion in medical practice when applying the detailed criteria to existing anticancer therapies and combination therapies with other anticancer agents. The HIRA plans to revise the 'Detailed Criteria and Methods for Applying Reimbursement (of Medications Prescribed·Administered to Cancer Patients),' including eligibility criteria, and implement it on June 1. Accordingly, at the CDRC meeting scheduled for May 14, they are expected to establish the reimbursable drugs and detailed criteria for partial reimbursement for combination cancer therapies and include them in the announcement of cancer drug reimbursement criteria set to take effect in June.
- Product
- Indiscriminate in-hospital prescriptions of Wegovy
- by Jung, Heung-Jun May 13, 2025 06:06am
- Criticism arose regarding the indiscriminate in-hospital prescriptions of Wegovy, which is currently allowed with an exception. According to the Korean Pharmaceutical Affairs Act, in-hospital prescription is allowed only when an 'injectable is injected.' However, it has been pointed out that Wegovy is being sold without a single act of injection. According to a local pharmacy, more hospitals have begun in-hospital prescriptions after Wegovy's non-face-to-face prescription was restricted as of December 2024. Pharmacists complain that "out-of-hospital prescriptions are being made only when hospitals run out of stock." In fact, it has been pointed out that proper monitoring has not been done despite frequent in-hospital prescriptions without meeting the requirement of 'when injected.' A Pharmacist 'A' in Seoul said, "Prescriptions are being made only when hospitals run out of stock and until restocked. We heard from patients coming to pharmacies that hospitals are selling the medication without giving injections," and added, "We are aware that in-hospital prescriptions are being restricted and doing so raises problem." A Pharmacist 'A' added, "Based on the guideline's recommendation, dosage must be initiated from Level 1, but there are cases where Level 2-3 are prescribed." Proper monitoring is deemed necessary because indiscriminate and misuse cases of in-hospital prescriptions were also found in hospitals and clinics that give injections. At a Wegovy online community with over ten thousand members, information on 'shared injection' cases in hospitals where a needle part of a single Wegovy is swapped and injected into multiple patients. Furthermore, supply price may differ, so when hospitals offer in-hospital prescriptions at a lower price, nearby pharmacies' selling prices are heavily affected. A Pharmacist 'B' in Seoul said, "Although we are seeing lower prescriptions compared to the early period of the launch, a few patients still visit. Due to the non-face-to-face platform, checking the Wegovy price got easier, and patients are sharing information online, so we have lowered the price at the pharmacy." Since Zuellig Pharma Korea does not directly transact with pharmacies, pharmacists purchase Wegovy through collaborative wholesale merchants, such as Kyungdongsa and Geo-young. Zuellig Pharma Korea reported that they have not changed the distribution price since the first domestic distribution in October 2024. It has been reported that there is a difference of about KRW 20,000 between the distributing price and that purchased through the collaborative wholesale merchants. Consequently, if pharmacies were to lower the selling price, adjusting to several hospitals that offer extremely low-priced in-hospital prescriptions, they must sell Wegovy at the buying cost.
- Company
- U.S. pharmaceutical imports rise twofold amid tariff concern
- by Kim, Jin-Gu May 13, 2025 06:05am
- U.S. drug imports have nearly doubled in a year. This is believed to be the result of frontline pharmaceutical and biotech companies focusing on securing inventory in expectation of U.S. President Donald Trump's drug tariff imposition. According to Korea Biotechnology Industry Organization (KoreaBIO) on the 12th, the U.S. The United States Census Bureau and the U.S. Bureau of Economic Analysis recently announced the results of U.S. trade in goods and services. According to the data, U.S. imports of goods in the first quarter totaled USD 997.8 billion. This is a 26.6% (USD 209.8 billion) increase from the USD 788 billion in the first quarter of last year. In particular, imports of pharmaceuticals increased significantly. In the first quarter, U.S. imports of pharmaceuticals amounted to USD 108.2 billion, a 97.2% (or USD 53.3 billion) increase from the USD 54.9 billion in the same period last year. Pharmaceutical imports surged in March during the first quarter. This coincides with the spread of concerns over President Trump's pharmaceutical tariffs. In fact, U.S. pharmaceutical imports, which stood at USD 29.5 billion in February, rose to USD 50.4 billion in March, an increase of 70.9% in just one month. Considering that total imports increased by 5.5% (from USD 326.5 billion to USD 344.3 billion) during the same period, the increase in pharmaceutical imports was particularly notable. The share of pharmaceuticals in total U.S. imports rose from 9.0% in February to 14.6% in March, an increase of 5.6 percentage points in a single month. KoreaBIO explained that the increase in U.S. pharmaceutical imports was due to the proactive response of local pharmaceutical and biotech companies to concerns over President Trump's import tariffs. KoreaBIO analyzed that “local pharmaceutical and biotech companies appear to be focusing more on securing inventory in the U.S. this year as a short-term response.” Domestic companies are also focusing on securing inventory in the U.S. As of the 7th of this month, Celltrion has completed the transfer of approximately 15 months' worth of inventory for products scheduled for sale in the U.S. Celltrion expects that this will minimize the impact of tariffs not only this year but also in the first half of next year. Celltrion plans to minimize risk in the mid- to long-term by producing finished drug products (DP) through local contract manufacturing organizations (CMOs) in the US. The company explained that it has completed negotiations to secure additional quantities in preparation for the possibility of increased tariff burdens after next year. At the same time, it is pushing ahead with securing active pharmaceutical ingredient (API) manufacturing facilities in the US. It has completed preliminary reviews and has entered the stage of detailed reviews. A Celltrion official said, “We have been devising various strategies in advance to respond to the possibility of changes in President Trump's tariff policy,” adding, “Once the policy is finalized, we will promptly share a comprehensive response plan with our shareholders.” The U.S. government plans to make a separate announcement on whether to impose tariffs on pharmaceuticals. Although it was not included in the mutual tariffs announced on the 2nd of last month, expectations are growing that separate tariffs on specific items will be imposed in May. Prior to this, the U.S. Department of Commerce has been assessing the impact of imports of pharmaceuticals and pharmaceutical raw materials on national security under Section 232 of the Trade Expansion Act, and requested public comments from interested parties from the 16th of last month to the 7th of this month. It is reported that a total of 957 companies and institutions worldwide, including the United States, submitted public comments. South Korea also submitted comments through the government, KoreaBIO, and the Korea International Trade Association(KITA). U.S. companies and institutions, including the Pharmaceutical Research and Manufacturers' Association, are said to have submitted comments stating that “imposing tariffs on pharmaceuticals is not the answer.”