- LOGIN
- MemberShip
- 2025-12-22 13:03:47
- Company
- 'Onbevezy' holds the No.1 spot for the domestic mkt
- by Chon, Seung-Hyun Mar 26, 2025 06:00am
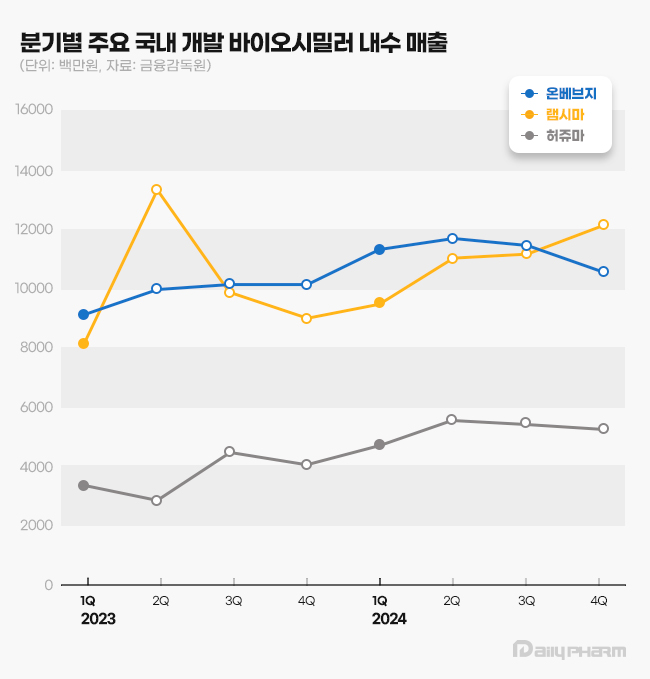
- Samsung Bioepis' anticancer agent Onbevezy climbed to hold the No.1 spot in domestic sales as the first domestically developed biosimilar. It has surpassed Remsima for the first time. The quarterly sales of Onbevezy and Remsima exceeded KRW 10 billion, each competing to hold the No.1 spot. According to the Financial Supervisory Service on March 26, Samsung Bioepis' Onbevezy generated domestic sales of KRW 45.2 billion last year, up 14.0% from the previous year. Onbevezy chased Remsima's sales last year, KRW 44 billion, by a difference of KRW 1.2 billion, and rose to hold the No.1 spot for domestic sales of domestically developed biosimilars. Domestic sales of Korea-made biosimilars by years (unit: 1 million, source: Financial Supervisory Service). Legend: Blue-Onbevezy, Yellow-Remsima, Gray-Herzuma Onbevezy is a biosimilar to the anticancer drug Avastin. It is an anticancer drug used to treat patients with metastatic colorectal cancer, metastatic breast cancer, non-small cell lung cancer, advanced or metastatic renal cell carcinoma, glioblastoma, epithelial ovarian cancer, carcinoma of the fallopian tube, primary peritoneal cancer, and cervical cancer. Samsung Bioepis launched Onbevezy in September 2021 in the Avastin market. Celltrion and Alvogen Korea also entered the market. In 2023, Onbevezy was behind sales of Remsima by KRW 800 million, but it successfully exceeded Remsima sales last year. Onbevezy entered the market first among other biosimilar products. Analysis suggests that a customized marketing strategy maximized the synergy. After obtaining the domestic approval of Onbevezy, Samsung Bioepis signed an exclusive sales agreement with Boryung, one of the Korean companies with strength in the field of anticancer drugs. In May 2020, Boryung newly established the ONCO (anticancer) sector. The company independently separated the sector from the prescription medicine sector. Boryung secured sales rights to various anticancer drugs and biosimilars owned by Korean and international companies. With the LBA (Legacy Brands Acquisition) strategy of acquiring sales rights for original anticancer drugs, Boryung secured Gemzar and Alimta. Boryung also secured Korean sales rights for Herceptin biosimilar from Samsung Bioepis. In Q2 2023, Onbevezy surpassed sales of KRW 10 billion, and the drug also recorded quarterly sales of over KRW 10 billion. Remsima, which Celltrion sells, generated sales of KRW 44 billion last year, up 8.7% from the previous year. However, it allowed Onbevezy to overtake its position. Remsima is a biosimilar to the autoimmune disease treatment Remicade. It was approved as the first domestically developed antibody biosimilar in 2012. Remsima is used to treat Crohn’s disease, ankylosing spondylitis, ulcerative colitis, and rheumatoid arthritis. Upon launch, Remsima continued to generate the highest domestic sales figure for over 10 years among domestically developed biosimilars in South Korea. Remsima generated global sales of KRW 1.2680 trillion last year, an increase of over three-fold from KRW 420 billion in 2023. It topped KRW 1 trillion in yearly sales, first among domestically developed pharmaceuticals. Domestic sales of Korea-made biosimilar by quarters years (unit: 1 million, source: Financial Supervisory Service) Legend: Blue-Onbevezy, Yellow-Remsima, Gray-Herzuma Quarterly sales figures indicate that Remsima lost the No.1 rank for five consecutive quarters through Q3 of last year after allowing Onbevezy to overtake the position in 2023. However, in Q4 of last year, Remsima reversed the trend again, indicating strong competition for the top position. In Q4 of 2024, Remsima's sales reached KRW 12.1 billion, a 35.3% increase year-over-year (YoY), outpacing Onbevezy by KRW 1.6 billion. In contrast, Onbevezy's Q4 sales for last year were KRW 10.6 billion, a YoY increase by 4.3%. However, Onbevezy lost to Remsima in just six quarters. The introduction of Korea-made biosimilars typically drives down the prices of original drugs, thereby reducing the burden on the National Health Insurance budget. Following the listing of Onbevezy, in October 2021, the ceiling price for Avastin 0.1 g/4 mL was reduced by 30%, from KRW 330,387 to KRW 231,271, and that for Avastin 0.4 g/16 mL dropped by 30% from KRW 1,077,531 to KRW 752,746. According to the policy, when a biosimilar enters the domestic market, the ceiling price for the original drug is lowered by 30% compared to its pre-patent-expiration level. However, 'Products developed by innovative pharmaceutical companies·Those deemed equivalent·products developed through joint agreements between domestic and foreign pharmaceutical firms·Products for which Korea was the first approving country·Products manufactured domestically' are guaranteed to retain up to 80% of the original product's pre-patent-expiration price for both the original drug and its biosimilar. Since Samsung Bioepis is not classified as an innovative pharmaceutical company, Avastin's price fell to about 70% of its previous level. According to IQVIA, a pharmaceutical market research firm, Avastin recorded sales of KRW 30.9 billion in Q3 2021, but it declined sharply to KRW 22.6 billion in Q1, a drop of 27.2%. The introduction of biosimilars, which led to a 30% reduction in the original drug's price, is analyzed to have resulted in significant savings for the National Health Insurance budget and patients' drug costs. Among Korea-made biosimilars, Celltrion's Herzuma generated sales of KRW 21.3 billion last year, an increase of 43.4% from the previous year. Herzuma is a biosimilar version to Herceptin. Truxima, Celltrion's biosimilar version of MabThera, generated sales of KRW 11.4 billion last year, a drop of 23.2% from the year before.
- Company
- Will topical JAK inhibitors be launched?
- by Son, Hyung Min Mar 25, 2025 05:57am
- Topical formulation of Janus kinase (JAK) inhibitors with the substantial advantage of administration convenience is entering the market for atopic dermatitis. Unlike oral formulations, a topical drug formulation can be directly applied to the skin. Thus, it has significantly improved treatment convenience. To date, Incyte's Opzelura is the only JAK inhibitor approved by the U.S. Food and Drug Administration (FDA). Leo Pharma's delgocitinib is waiting for approval. HK inno.N has entered the Phase 3 trial in South Korea. HK inno.N is conducting a Phase 2 trial of topical JAK inhibitor…first in South Korea According to industry sources on March 22, the Ministry of Food and Drug Safety (MFDS) has recently approved an Investigational New Drug (IND) application of the Phase 2 trial of HK inno.N's new drug candidate, 'IN-115314.' The clinical trial will evaluate the efficacy and safety of IN-115314 in adult patients with mild-to-moderate atopic dermatitis. The trial's clinical research organization is the Korean University Ansan Hospital, and it will be investigated by Professor Sang Wook Son in the Department of Dermatology. IN-115314 is a topical JAK inhibitor under development by HK inno.N. This new drug candidate works by topically applied to the inflammatory areas and selectively inhibits JAK-1 kinase. HK inno.N believes that IN-115314 has a lower amount of whole-body absorption than conventional drugs, thus presenting a low risk of side effects. JAK inhibitors that are currently approved have various indications, including atopic dermatitis, ulcerative colitis, rheumatoid arthritis, and alopecia areata. Considering the nature of externally applied drugs, companies focus on developing skin disorder products. Notably, domestically approved JAK inhibitors, including AbbVie's Rinvoq, Lilly's Olumiant, Pfizer's Xeljanz, and Eisai's Jyseleca, have been developed exclusively as oral formulations. Consequently, a topical formulation could offer significant advantages regarding dosing convenience. In a Phase 1 clinical trial involving healthy adults, IN-115314 demonstrated favorable safety, tolerability, and pharmacokinetic profiles compared to the calcineurin inhibitor 'pimecrolimus' ointment, which is conventionally used in atopic dermatitis treatment. HK inno.N plans to conduct a Phase 2 clinical trial as a correct-dose finding study in adult patients with atopic dermatitis. Topical JAK inhibitor successfully commercialized…follow-up products are being actively developed Several topical JAK inhibitors overcome regulatory authority hurdles, so overseas companies are actively developing products. IncyteIn 2021, the FDA approved U.S.-based Incyte's 'Opzelura' for the treatment of atopic dermatitis. The active ingredient ruxolitinib in Opzelura cream was previously used under the product name Jakafi for treating cancer patients with conditions such as myelofibrosis, polycythemia vera, and chronic graft-versus-host disease. Incyte successfully developed Opzelura as a treatment for atopic dermatitis based on Jakafi's JAK inhibitory mechanism. Opzelura has been shown to alleviate inflammation and pruritus when applied topically in atopic dermatitis patients aged 12 and older. In 2022, Insight secured U.S. approval for Opzelura not only for atopic dermatitis but also for vitiligo. Clinical development of Opzelura is ongoing, with Incyte currently conducting a Phase 3 trial in infants and children with atopic dermatitis. The trial met its primary endpoints, including overall treatment success as assessed by the investigators. Denmark's pharmaceutical company, LEO Pharma, has entered the competitive market by unveiling the latest clinical data for its topical JAK inhibitor delgositinib. The company, specializing in dermatological drug development, successfully commercialized the biologic treatment 'Adtralza' for atopic dermatitis. LEO PharmaLEO Pharma is developing a topic formulation of delgositinib that targets JAK1, JAK2, JAK3, and TYK2. TYK2 plays a critical role as a central link in the interleukin (IL)‑23 signaling pathway, which is pivotal in developing skin disorders such as psoriasis. Last November, delgositinib received European marketing authorization under the brand name 'Anzupgo' for the treatment of chronic hand eczema (CHE), and it is currently awaiting FDA approval. Delgositinib demonstrated exceptional improvement in clinical studies in patients with moderate-to-severe chronic hand eczema. According to research presented at the American Academy of Dermatology earlier this month, in the clinical trials designated DELTA 1 and DELTA 2, 48% of patients achieved a deep response within 16 weeks, over 24% exhibited a consistent response, and 33% maintained their response even after discontinuing treatment. Professor April Armstrong, affiliated with the David Geffen School of Medicine at UCLA, who presented the clinical results, said, "Delgositinib demonstrated approximately threefold higher efficacy than conventionally used treatments," adding, "Notably, the trial achieved significant advancement in difficult-to-treat patients with CHE."
- Company
- Adempas’s nears reimb nearly 10 years after approval
- by Eo, Yun-Ho Mar 25, 2025 05:54am
- The reimbursement of the pulmonary arterial hypertension drug 'Adempas' is near in Korea, 10 years after its approval. The National Health Insurance Service is currently negotiating with Bayer Korea for its Adempas (riociguat). However, the negotiations are expected to be concluded and the drug listed soon. The negotiations for Adempas are not over its insurance ceiling price but about its amount of use. Bayer accepted a price below 100% of the weighted average price (WAP) of its alternative drug and passed review by the Health Insurance Review and Assessment Service’s Drug Reimbursement Evaluation Committee in February. In other words, it is a drug that is eligible for the ‘drug price negotiation exemption.’ As a result, the drug is likely to be listed in the first half of the year. Adempas was approved in Korea as an orphan drug in June 2014 and is available in 5 dosage forms. It is indicated for: ▲Improvement of exercise capacity in adult patients with chronic thromboembolic pulmonary hypertension (CTEPH, WHO Group 4) who are unable to undergo surgery or who have persistent or recurrent symptoms after surgery ▲Improvement of exercise capacity in adult patients with pulmonary arterial hypertension (WHO Group 1) who are classified as having functional class II or III. In particular, it was known as the first new drug for CTEPH. CTEPH is caused by patients who develop chronic pulmonary embolism, which leads to fibrotic stenosis and occlusion, resulting in pathological vascular remodeling and increased resistance in the pulmonary artery. CTEPH is a chronic disease that causes progressive dyspnea and right heart dysfunction, which weakens the heart. Symptoms include dyspnea, fatigue, chest pain, dizziness, peripheral edema, cough, and hemoptysis, which reduces the patient’s quality of life. Ultimately, it can progress to heart, kidney, and liver failure, which can lead to death. Meanwhile, Adempas is a stimulator of soluble guanylate cyclase (sGC), an enzyme found in the heart and lungs, and its efficacy has been confirmed in two Phase III clinical trials in patients with chronic thromboembolic pulmonary hypertension. Results showed improvement in exercise capacity, which was the primary endpoint, and good tolerability. No unexpected adverse reactions were reported. In the CHEST-1 study, when comparing the 6-minute walking test (6MWT) results after 16 weeks with the baseline, results showed that the group of patients who received riociguat showed statistically significant improvement compared to the group of patients who received placebo. In the PATENT-1 study, the change in the 6MWT score after 12 weeks of treatment, showed statistically significant improvement in the riociguat arm compared to placebo, meeting the primary endpoint.
- Company
- HLB shares plummet upon 2nd FDA rejection of rivoceranib
- by Cha, Jihyun Mar 25, 2025 05:54am
- The shares of HLB Group affiliates plummeted as HLB's new drug for liver cancer failed to enter the US market again. The total market capitalization of HLB Group stocks evaporated by over KRW 3 trillion in a single day. However, this did not cause a simultaneous drop in domestic bio stocks. HLB Group's 10 listed affiliates evaporate by KRW 3 trillion in a single day upon receiving a second Complete Response Letter (CRL) According to the Korea Exchange on the 22nd, HLB closed at KRW 46,500 on the 21st. This is a 29.97% drop from the previous trading day. The day before, HLB's share price plunged to the price floor immediately after the opening and remained at this price until the closing time. The market capitalization evaporated by KRW 2.6147 trillion in a single day as the stock price hit the floor. HLB's market capitalization was KRW 8.7241 trillion based on the closing price on the 20th, but it plunged to KRW 6.1095 trillion based on the closing price on the 21st. HLB's share price fell sharply after its new drug for liver cancer failed to enter the US market. HLB said on its official YouTube channel at 3 a.m. on the 21st that it had received a CRL from the US Food and Drug Administration (FDA) for its rivoceranib+camrelizumab. In May last year, the company received a CRL completed the supplementary work, and submitted the required documents for a second review, but received another CRL. Upon the news of HLB’s CRL receipt was announced, the share prices of HLB Group’s stocks fell across the board. HLB Global, HLB Life Sciences, HLB Pharma, and HLB PanaGene all recorded the lowest opening price on the 21st. The opening prices of HLB affiliates on the 21st were KRW 2,535 for HLB Global, KRW 5,990 for HLB Life Sciences, KRW 17,100 for HLB Pharma, and KRW 1,665 for HLB PanaGene, down 29% from the previous trading day. HLB bioStep and HLB Innovation also opened at a price 26% lower than the previous day's closing price. HLB Genex and HLB Science opened at a price 21% and 15% lower than the previous day's closing price. The ten HLB Group’s listed affiliates showed a downward trend throughout the day. HLB (-29.97%), HLB Life Sciences (-29.94%), and HLB Pharma (-29.92%) closed at the floor price. HLB Global (-18.09%), HLB Genex (-15.54%), HLB Science (-14.95%), HLB bioStep (-14.71%), HLB PanaGene (-14.32%), HLB Therapeutics (-7.37%), and HLB Innovation (-6.60%), also closed at a low price. As a result, KRW 3.3226 trillion of the total market capitalization of HLB Group shares was lost in a single day. As of the closing price on the 20th, the market capitalization of the 10 listed affiliates of HLB Group totaled KRW 12.924 trillion. As of the closing price on the 21st, the total market capitalization of these affiliates was KRW 8.7698 trillion. As of the closing price on the 21st, KRW 8 trillion of the market capitalization of HLB Group stocks had evaporated compared to the end of last month. The stock prices of HLB Group's listed affiliates suddenly soared on the 27th of last month. The analysis was that the rise was the result of the combined expectations for new drug approvals and the successive stock purchases of HLB Group Chairman Yang-gon Jin, the owner. At the time, the total market capitalization of HLB Group stocks reached KRW 16.5843 trillion. A similar situation occurred last year when the company received its first CRL. The total market capitalization of the 9 HLB Group’s listed affiliates, excluding HLB Genex, which the company acquired at the end of last year, fell by KRW 5.274 trillion from the previous day on May 17 last year, when the news of the failure to obtain approval for the use of rivoceranib+camrelizumab was announced. At an online press conference on the morning of the 21st regarding the CRL notification, Chairman Yang-gon Jin said, “I think that the shareholders as well as our employees at HLB Group are disappointed by the receipt of this CRL. I would like to express my regrets about this and will actively communicate through meetings after the shareholders' meeting.” However, HLB’s situation did not affect the domestic bio-sector. On the 21st, Sam Chun Dang Pharm, Kolon TissueGene, and LigaChem Biosciences showed a strong trend despite the news of the CRL for rivoceranib+camrelizumab. On the 21st, the closing price of Sam Chun Dang Pharm was KRW 188,800, up 7.64% from the previous day. The closing prices of Kolon TissueGene and LigaChem Biosciences on the 21st also rose 6.88% and 1.72%, respectively, from the previous day. Peptron (+4.29%), PharmaResearch (+2.35%), and Hugel (+1.21%) also closed up. This is in contrast to a past case in which the failure of a company to receive approval for a new drug dampened investment sentiment in the entire biotech industry. Domestic biotech stocks fell in tandem shortly after the announcement of the suspension of the clinical trial of SillaJen's liver cancer treatment 'Pexa-Vec' in 2019 and the failure of its clinical trial on Helixmith's diabetic neuropathy (DPN) gene therapy ‘Engensis' (VM202) in 2020. As a result, investor sentiment froze, and biotech stocks experienced dark ages for a while. Acquired from Bukwang Pharmaceutical in 2018, final data released at ASCO last year demonstrated OS extension Rivoceranib is an oral targeted anticancer drug of the vascular endothelial growth factor receptor 2 (VEGFR2) inhibitor class, which is involved in the formation of new blood vessels in tumors. The drug was developed in 2005 when US-based Elevar Therapeutics bought the global rights to rivoceranib from the Advenchen Laboratories. Bukwang Pharmaceutical, which recognized the potential of rivoceranib, secured the rights to sell the drug in Korea, Europe, and Japan from Elevar Therapeutics in 2009. HLB Life Sciences acquired the development rights to rivoceranib from Bukwang Pharmaceutical in 2018 for KRW 40 billion. HLB then acquired the product patent rights for rivoceranib in 2020 and capitalized on it as its material. HLB has been developing a rivoceranib+camrelizumab combination therapy with Jiangsu Hengrui Pharmaceuticals as a treatment for liver and stomach cancer. Camrelizumab, which was developed by Jiangsu Hengrui Pharmaceuticals, is an immuno-oncology drug that inhibits the PD-1 protein expressed on the surface of immune cells (T cells), preventing them from binding to the PD-L1 receptor on the surface of cancer cells and activating immune cells. HLB applied for an NDA to the FDA in May 2023 for the rivoceranib+camrelizumab combination as a first-line treatment for liver cancer. The results of the Phase III CARES-310 study, which was presented by HLB and Jiangsu Hengrui Pharmaceuticals at the 2022 European Society for Medical Oncology (ESMO) Congress, were presented as grounds for its approval. The clinical trial was conducted to compare the efficacy and safety of lenvatinib and camrelizumab with that of Bayer's Nexavar, which is the current standard of care for liver cancer. In the study, rivoceranib+camrelizumab recorded a median overall survival (mOS) of 22.1 months, showing improved results compared to Nexavar’s 15.4 months. This result showed a longer OS than the 19.2 months found with Roche's combination therapy of the immuno-oncology drug Tecentriq and the targeted-oncology drug Avastin, which has been approved as a first-line treatment for liver cancer, and the OS of 16.4 months for AstraZeneca's combination therapy of the immuno-oncology drug Imfinzi and Imjudo. The clinical trials for the combinations comparing the respective combinations with Nexavar monotherapy. The progression-free survival (PFS) of rivoceranib+camrelizumab was 5.6 months, and the objective response rate (ORR) was 33.1%. HLB also released final results that were even better than the clinical phase III trial results previously announced at the American Society of Clinical Oncology (ASCO) meeting last year. According to the results announced by HLB at the ASCO Annual meeting in May last year, the OS of rivoceranib+camrelizumab was 23.8 months, which was longer than the previous results of 22.1 months. HLB has included the additional improved data during re-submission to the FDA.
- Company
- The first RSV vaccine 'Arexvy' to launch in May in Korea
- by Whang, byung-woo Mar 25, 2025 05:54am
- As the launching date of Arexvy, known as the first respiratory syncytial virus vaccine, has been announced, the company aims to challenge a market share. Product photo of ArevyAccording to industry sources on March 22, GSK Korea has confirmed the launching date of the RSV-LRTD vaccine, Arexvy, as May. Arexvy received approval from the Ministry of Food and Drug Safety (MFDS) at the end of December 2024 for the 'Prevention of lower respiratory tract disease (LRTD) caused by RSB in adults over 60 years of age and older.' Approval of Arexvy was based on results from two Phase 3 studies, 'RSV OA=ADJ-006' and 'RSV OA=ADJ-004,' involving adults 60 years of age and older. The study results showed that during the first RSV season, Arexvy significantly lowered the RSV-LRTD risk by 82.6% and severe RSV-LRTD risk by 94.1% in participants 60 years of age and older compared to placebo. Furthermore, the efficacy of the vaccine regarding RSV-A-associated LRTD increases and RSV-B-associated LRTD increases were 84.6% and 80.9%, respectively. Arexvy has quickly secured the rank as the blockbuster product in the global market based on its first-in-class RSV vaccine title. The drug's influence in South Korea is gaining attention. If Arexvy launches in May, vaccination using the drug will likely begin in June, considering hospital landing settings. The remaining issue is how much the health authority considers RSV disease burden in seniors. Health authorities in major countries recommend RSV vaccination. However, the Center For Disease Control (CDC)'s Advisory Committee on Immunization Practices (ACIP) has narrowed the scope of vaccination. Previously, ACIP recommended vaccination in all adults of 60 years and older after consulting doctors. The ACIP has recently reduced the scope to adults 75 years and older or adults 60 to 74 years who have a high possibility of developing severe symptoms. In South Korea, some view that people would be less interested in Arexvy than the antibody injection for young children, Beyfortus (nirsevimab). Professor A from a department of infectious disease at one of the tertiary general hospitals in Seoul said, "Theoretically, RSV may likely affect those who are immune-compromised or older age. However, vaccines may not likely gain attention like those for newborns and young children," adding, "An additional investigation and evaluation may be needed to determine the potential impact since an adequate evaluation on disease burden has not been thoroughly established yet." In other words, even if a RSV vaccine targeting seniors are released, it may not immediately expand market compared to those targeting young children. Yet, potential demand may increase after the introduction of vaccine, as there has been little interest in the RSV vaccine for seniors. Considering these factors, GSK Korea will likely focus on raising awareness of RSV prevention along with Arexvy's launch. Hyunji Kwon, GSK Korea's Vaccine Business Unit Head, said, "RSV infection poses a significant physical and economic burden on high-risk groups, such as seniors. GSK will put efforts into preventing adult infections and relieving the disease burden of Korean patients after the successful launch of Arexvy."
- Policy
- New law proposed for the cancer and rare disease fund
- by Lee, Jeong-Hwan Mar 25, 2025 05:54am
- A bill to establish a new fund for cancer and rare diseases to strengthen patient access to ultra-high-priced drugs has been proposed to the National Assembly. The fund will be raised through transfers and deposits from other funds, such as the lottery fund. On the 24th, National Assembly member Myeong-ok Seo (People Power Party), a member of the National Assembly's Health and Welfare Committee, announced that she had submitted a bill to establish the Cancer Management Fund and the Rare Disease Management Fund. The main points of the bill include ▲the establishment of a cancer management fund for the prevention and treatment of cancer (amendment to the Cancer Management Act), ▲the establishment of a rare disease fund for the prevention and treatment of rare diseases (amendment to the Rare Disease Management Act), ▲the establishment of a basis for the establishment of the fund in the National Finance Act (amendment to the National Finance Act), and ▲the establishment of grounds for the use of the lottery fund (amendment to the Lottery Tickets and Lottery Fund Act). According to the data that Rep. Seo received from the Health Insurance Review and Assessment Service, it took an average of 332 days for an anticancer drug to be listed for reimbursement in Korea from 2014 to 2024. In particular, in the case of anticancer drugs for blood cancer and lung cancer, there were cases where it took 600 to 800 days to listing. To address this, the UK operates an anticancer drug fund and a rare drug fund. Italy also has a rare disease drug fund that is financed with 5% pharmaceutical sales promotional expenses and government funds. Although there have been attempts to establish a new cancer management fund in Korea, the attempts have been frustrated by the opposition of financial authorities, with repeated calls on the need for realistic solutions to raise necessary finances. “Currently, about KRW 85 billion of the National Health Promotion Fund is being spent on cancer prevention and treatment, so it is not impossible to use it as the source of funding as it is while using a portion of the lottery proceeds, which have been increasing rapidly recently,” explained Seo. According to recent reports, lottery sales have risen by an average of KRW 450 billion per year since 2020. Next year, lottery ticket sales are expected to exceed KRW 8 trillion for the first time in history. “In a situation where it is becoming structurally difficult to apply health insurance reimbursement to treatments for patients with cancer and rare diseases, the establishment of the fund is a matter directly related to the lives of the people,” said Rep. Seo. “I hope that the bill will prompt forward-looking discussions.”
- Company
- 'Xeljanz' reimbursed for juvenile idiopathic arthritis
- by Eo, Yun-Ho Mar 24, 2025 05:52am
- Product photo of Xeljanz'Xeljanz' has become the first JAK inhibitor to be reimbursed for the treatment of juvenile idiopathic arthritis. The Ministry of Health and Welfare (MOHW) has recently announced on the administrative notification board regarding the 'The Criteria and Scope of National Health Insurance (Pharmaceuticals)' that the reimbursement criteria for Pfizer Korea's Xeljanz (tofacitinib) will be expanded from April. Xeljanz can be used to treat children (age 2 to 17) diagnosed with juvenile idiopathic arthritis according to the ILAR criteria (2001 revision), including ▲Polyarticular arthritis that affects five or more joints ▲Extended oligoarthritis ▲Psoriatic arthritis ▲Those who discontinued treatments due to inadequate response to one or more biological agent or side effects. After 6 months of usage, an additional 6-month usage will be approved if an assessment indicates a decrease of over 30% in the number of inflammatory joints compared to the initial administration timepoint. After that, the evaluation will be carried out every 6 months, and when the assessment result in the first 6 months is maintained, consistent administration will be approved. This reimbursement approval is the first among the JAK inhibitors. To date, 'Olumiant (baricitinib)' secured the same indication in September last year but is still non-reimbursed. Similarly, 'Rinvoq (upadacitinib)' has not been domestically approved for treating idiopathic arthritis. Following the patent expiration of Xeljanz, Pfizer has made efforts to increase product competitiveness by changing formulations in many ways. In 2020, the company launched an extended-release formulation with fewer administrations for various arthritis treatments. In 2023, Pfizer also launched a syrup formulation that is more convenient to administer to pediatric patients. It is to be watched whether Xeljanz, with an opportunity to take the market share, prescription will increase in the idiopathic arthritis area. Meanwhile, the efficacy of Xeljanz was demonstrated through the Phase 3 'JIA-I' trial. The study compared the effectiveness and safety of the drug to a placebo in 225 patients aged 2 years and above and those aged 18 years and below. In the study, Xeljanz tablet or syrup formulation (dosage depending on the weight range; 5 mg was administered less than twice a day) was administered for 18 weeks. Patients (142 individuals) who reached the JIA ACR 30 (symptom improvement over 30%) were divided into Xeljanz and placebo groups. The results at week 44 confirmed that the symptom worsening in the Xeljanz group (29%, 72 individuals) was significantly lower than those in the placebo group (53%, 70 individuals). During the same period, the rate of reaching the JIA ACR30∙50∙70 (30∙50∙70% symptom improvement) was higher in the Xeljanz group. Physical function measured by the Childhood Health Assessment Questionnaire (CHAQ) also confirmed significant improvement in the Xeljanz group (-0.11) compared to the placebo group (0.00).
- Company
- RSV vaccine Beyfortus lands in Big 5 Hospitals in Korea
- by Eo, Yun-Ho Mar 24, 2025 05:52am
- The respiratory syncytial virus (RSV) preventive antibody injection ‘Beyfortus’ has landed in the Big 5 tertiary hospitals in Korea. According to industry sources, Sanofi Korea's Beyfortus (nirsevimab) has passed the drug committees (DCs) of the Big 5 tertiary hospitals in Korea, including Samsung Medical Center, Seoul National University Hospital, Asan Medical Center, and Severance Hospital. In addition, the drug has also landed at medical institutions such as Gangnam Severance Hospital, Korea University Anam Hospital, Korea University Ansan Hospital, Bundang Severance Hospital, and Pusan National University Yangsan Hospital. Also, Beyfortus vaccination has begun at local clinics since February. SK Bioscience is in charge of Beyfortus’s promotional activities for medical institutions at the clinic level. Beyfortus is an RSV preventive antibody injection that received approval from the Ministry of Food and Drug Safety in May last year and can be administered to all newborns and infants who are entering their first RSV season. Also, children up to 24 months of age who remain at risk of severe RSV disease through their second RSV season may receive Beyfortus. Previously, RSV prevention products for infants and toddlers in Korea were only administered to high-risk infants and toddlers, such as premature babies, who are at high risk of severe RSV disease. However, Beyfortus is different in that it can be administered to all infants and toddlers. According to the Phase III MELODY trial, which was the basis for the approval of Beyfortus, RSV lower respiratory tract infections were reduced by 74.5% in the Beyfortus-administered arm. This study evaluated the efficacy of Beyfortus against RSV infection with medical management up to 150 days after administration in 3,012 infants born after 35 weeks of gestation in their first RSV season. In addition, according to the interim results of the national vaccination program being implemented in Galicia, Spain, real-world evidence of Beyfortus, hospitalizations due to RSV in infants under 6 months of age who received Beyfortus were reduced by 82% compared to infants who did not receive Beyfortus. Ki-Wook Yoon, a Professor at Seoul National University Hospital, said, “RSV can infect people of all ages, but 90% of infants under the age of 2 are infected. When infected, it can lead to mild cold symptoms to hospitalization due to lung infection. Infants whose bronchial tubes are not fully mature can have more severe symptoms when they are infected with RSV, which can cause losses not only for family members but also to the society and economy.” He added, “Until now, RSV prevention was limited to personal hygiene practices, showing a clear unmet demand for RSV. However, we expect that active RSV prevention will become possible with the introduction of the preventive antibody injection.”
- Policy
- Tepmetko, Tevimbra granted reimbursement in Korea
- by Lee, Tak-Sun Mar 24, 2025 05:52am
- Tepmetko Tab 225mg (tepotinib, Merck Korea). New anticancer drugs Tepmetko and Tevimbra will be included in the list of reimbursed drugs as of April 1 in Korea. In addition, the economic burden on the patients is expected to be significantly reduced as the co-insurance rate for abiraterone acetate drugs such as Zytiga has been reduced for the first-line treatment of castration-resistant prostate cancer. The Health Insurance Review & Assessment Service has announced a revision to the notice regarding the drugs prescribed and administered to cancer patients and has entered the opinion survey period. The effective date is April 1. According to the revision, Tepmetko 225 mg (tepotinib, Merck Korea) is granted reimbursement for patients with locally advanced or metastatic non-small cell lung cancer with MET exon 14 deletion. HIRA said, “We set the reimbursement criteria by considering factors such as the fact that the applied drug is a drug with a clear target and can provide patients with another treatment option, and is a drug deemed necessary for medical treatment.” Tepmetko is the only MET-mutated anticancer drug that is covered by the National Health Insurance in Korea. According to the diagnosis of 1,020 patients with non-small cell lung cancer in Korea, 1.9% of patients were confirmed to have MET exon 14 deletion. In Phase II clinical trial which added a confirmatory test arm, Tepmetko showed an ORR of 51.4% (95% CI, 45.8-57.1), mPFS of 11.2 months (95% CI, 9.5-13.8), and mOS of 19.6 months (95% CI, 16.2-22.9), confirming its high therapeutic effect. This drug is recommended in major textbooks and guidelines of overseas academic societies. The insurance ceiling price of Tepmetko is reportedly KRW 76,500 per dose. It has signed a refund-type and expenditure cap-type Risk Sharing Agreement (RSA) to share the drug’s financial burden. Tevimbra Inj (tislelizumab, BeiGene Korea) is indicated as a monotherapy for patients with unresectable, relapsed, locally advanced, or metastatic oesophageal squamous cell carcinoma who are unable to continue platinum-based chemotherapy or who have relapsed or progressed within 6 months after receiving prior platinum-based chemotherapy. However, reimbursement is granted for patients who have not received treatment with an immune checkpoint inhibitor such as a PD-1 inhibitor. As an immuno-oncology drug that has a PD-1 inhibitory mechanism of action, its reimbursement listing in April will allow the drug to become the first immuno-oncology drug to be covered for esophageal cancer in Korea. The maximum amount of this drug is KRW 1,206,000 per bottle. Like Tepmetko, it is applied the refund type and expenditure cap type RSA. Meanwhile, the co-insurance rate for abiraterone acetate formulations such as Zytiga will be reduced from 30% to 5% for the first-line treatment of castration-resistant prostate cancer. Abiraterone is available not only as the original Zytiga (Janssen Korea) but also as generic versions supplied by Hanmi Pharmaceutical and Ace Pharmaceutical. This reduction in the co-insurance rate is also related to the entry of generics. The National Health Insurance Service said, “The reimbursement of the ‘next-generation hormone drug (all-trans retinoic acid, ATRA)’ for metastatic hormone-sensitive prostate cancer will gradually decrease the number of patients eligible for this treatment. The price of the drug has been reduced upon the listing of generics, etc, and the coinsurance rate for ‘abiraterone acetate + prednisolone’ as a first-line treatment for castration-resistant prostate cancer will be reduced from 30% to 5%." The ATRA-class next-generation hormone drug is Janssen's Erleada. Erleada was listed for reimbursement in April 2023.
- Policy
- How Tevimbra was reimbursed in Korea first
- by Lee, Tak-Sun Mar 24, 2025 05:52am
- BeiGene Korea's Tevimbra will be reimbursed in Korea from next month as a second-line treatment for esophageal squamous cell carcinoma, a type of esophageal cancer. It is the first immuno-oncology drug to be covered for esophageal cancer. In particular, the drug is drawing attention as it was covered in South Korea before the A8 countries (the United States, the United Kingdom, Germany, France, Italy, Switzerland, and Japan) known as the government’s reference countries for reimbursement coverage. The reason why the drug received prompt reimbursement in Korea is that the insurance authorities have taken into account Korea’s situation, where the prevalence of esophageal cancer is relatively higher than in other countries, and that the pharmaceutical company, BeiGene Korea, also presented a reasonable drug price. According to industry sources on the 21st, Tevimbra (tislelizumab, BeiGene Korea) will be reimbursed starting next month for unresectable, recurrent, locally advanced, or metastatic esophageal squamous cell carcinoma that has relapsed or progressed during or after previous platinum-based chemotherapy. It is the first PD-1 inhibitor-based immuno-oncology drug to be covered by the National Health Insurance for esophageal cancer. Squamous cell carcinoma accounts for the largest proportion of esophageal cancer, accounting for 91% of esophageal cancer cases. In particular, its prevalence is reportedly higher in East Asia compared to that in North American and European countries. Its prognosis is also poor. Most cases are detected when the disease has progressed to a significant degree, so the survival rate is not high. According to data from the Korea Central Cancer Registry, the 5-year relative survival rate for esophageal cancer from 2017 and 2021 was only 42.8%. Platinum-based chemotherapy is mostly used as the first-line treatment for esophageal cancer, and docetaxel is used as the second-line or later treatment. Compared to existing anticancer drugs, immuno-oncology drugs have shown significant improvements in overall survival but are not widely used because they are not reimbursed by the National Health Insurance. Due to this situation, the health authorities reportedly have given high scores for Tevimbra in terms of its clinical utility. Experts at the Drug Reimbursement Evaluation Committee said, “Compared to the chemotherapy group, Tevimbra showed a significant improvement in overall survival and is safer with a lower risk of side effects than chemotherapy. Currently, there are no immuno-oncology drugs reimbursed for esophageal squamous cell carcinoma in Korea, and due to the many limitations in treatment options, Tevimbra’s introduction is necessary.” Such expert opinions were heavily reflected in the review of the drug's adequacy of reimbursement. “The fact that the number of esophageal cancer patients in Korea is higher than in the US and Europe and that the prognosis is poor was taken into consideration,” said a HIRA official. “It was difficult to assess the drug because it was not listed for reimbursement in the A8 countries and was not mentioned in major reference textbooks, but the review was conducted with a focus on the drug’s clinical aspects.” Of course, there were results from clinical studies that showed significant improvements in overall survival and progression-free survival, but there were relatively few references to textbooks or other such materials. However, there was a clinical practice guideline - the NCCN guidelines in the United States – which recommended the drug as a second-line or higher monotherapy. The company’s proposal of a reasonable drug price was also granted for the drug’s prompt reimbursement. BeiGene Korea was recognized for its cost-effectiveness by submitting the results of a pharmacoeconomic evaluation compared to docetaxel and the results of a cost-utility analysis using RSA plans (refund and expenditure cap type). BeiGene Korea has announced that it will offer a low drug price to ensure the rapid supply of Tebipembrolizumab. It is reported that the drug will be supplied at a 10% discount from existing treatments in the United States. Currently, the only immuno-oncology drugs that are undergoing the reimbursement process for esophageal cancer are Keytruda and Opdivo. They have been approved as first-line treatments, not second-line treatments. However, unlike these drugs, Tevimbra has the advantage of being able to be used regardless of PD-L1 expression. Tevimbra is an anticancer drug developed in China in 2019. The domestic approval was granted in November 2023, and the drug was successfully listed for reimbursement in 1 year and 4 months. Its price is KRW 1,206,000 per bottle, and it is expected to cost about KRW 9.65 million per year. If the 5% co-insurance rate is applied, the annual drug cost for the patients will be reduced to about KRW 480,000, which is a relatively low price for an immuno-oncology drug.