- LOGIN
- MemberShip
- 2025-12-22 13:08:39
- Company
- K-pharma trials show greenlight overcoming bile duct cancer
- by Son, Hyung Min Apr 04, 2025 05:58am
- South Korean and the overseas pharmaceutical industry's candidate products that are targeted anticancer agents for bile duct cancer (cholangiocarcinoma) are showing results of effectiveness, indicating the potential for commercialization possibilities. Korean and overseas pharmaceutical companies, including Handok's US partner Compass Therapeutics and HLB, are challenging this area. Compass "tovecimig met primary endpoint in global clinical trials" #iAccording to industry sources on April 3, US-based Compass announced top-line results from tovecimig's COMPANION-002 Phase 2/3 trial evaluating patients with metastatic or recurrent cholangiocarcinoma. Tovecimig is a novel drug candidate for cholangiocarcinoma, which was developed by the Korean company ABL Bio. Handok holds domestic commercialization rights, and Compass holds global rights. This new drug candidate is a dual antibody that simultaneously targets delta-like ligand 4 (DLL4) and vascular endothelial growth factor (VEGF), thereby inducing neovascularization in the tumor microenvironment. The study enrolled 168 adult patients with metastatic or recurrent cholangiocarcinoma and compare the efficacy and safety of the tovecimig+paclitaxel combination therapy versus paclitaxel monotherapy. The clinical trial results showed that the primary endpoint, objective response rate (ORR), was 17.1% in the tovecimig+paclitaxel group, compared to only 5.3% in the paclitaxel monotherapy group. Furthermore, the incidence of progressive disease (PD) was 16.2% in the combination group versus 42.1% in the monotherapy group. Regarding safety, Grade ≥3 adverse events observed in the Phase 2 trial were consistent with previous studies. These included neutropenia (50%), hypertension (16.7%), anemia (12.5%), and thrombocytopenia (8.3%). There was one reported case of Grade 5 pneumonia, and 25% of patients discontinued treatment due to adverse events such as confusion, pulmonary embolism, and elevated blood creatinine levels. Handok plans to utilize these results as supporting data for regulatory approval of tovecimig in Korea. Following these top-line findings, Compass intends to present additional data, including key secondary endpoints, from the COMPANION-002 trial later this quarter. Compass also supports a researcher-led clinical trial evaluating tovecimig as a first-line therapy for cholangiocarcinoma, in addition to the ongoing COMPANION-002 study. This trial, led by the MD Anderson Cancer Center at the University of Texas, investigates the efficacy and safety of adding tovecimig to the standard regimen of Imfinzi plus chemotherapy. Rivoceranib shows potential in cholangiocarcinoma Cholangiocarcinoma is considered one of the most challenging solid tumors due to its low survival rate and the limited availability of new targeted therapies. Although the patient population is relatively small compared to other cancers, early diagnosis remains difficult, and the disease is characterized by rapid metastasis and recurrence to surrounding organs, resulting in a 5-year relative survival rate of only 28.9% (2017–2021). Reports indicate that 7 out of 10 cholangiocarcinoma patients eventually die, and the domestic mortality rate in Korea is estimated at 11.6%. Another reason for low survival rate is the scarcity of effective treatment options. For patients with locally advanced or metastatic cholangiocarcinoma who are not candidates for surgery and have failed first-line therapy, there is a critical lack of second-line options. Although the development of various targeted therapies was possible for cholangiocarcinoma, like lung cancer, limited patient numbers have restricted research and investment. However, growing interest from the pharmaceutical industry is now yielding promising research outcomes. HandokHandok has recently expanded the therapeutic landscape by introducing two targeted treatments in Korea: its FGFR2-targeted therapy 'Pemzayre' and Servier’s IDH1-targeted therapy 'Tipsovo.' Other Korean and overseas companies are venturing into this field. FGFR genetic abnormalities are known to contribute not only to cancer cell proliferation, survival, and migration but also to tumor angiogenesis and drug resistance. Meanwhile, IDH1 mutations are predominantly found in gliomas and cholangiocarcinomas, with a particular prevalence in intrahepatic cholangiocarcinoma. In addition to Handok, Korean company HLB is actively investigating new treatment options for cholangiocarcinoma. HLB, in collaboration with Hangseo Pharmaceuticals, is evaluating the clinical efficacy of a combination regimen comprising the VEGFR2 inhibitor riboceranib and the immuno-oncology agent camrelizumab in several solid tumors, including liver cancer and cholangiocarcinoma. In a clinical study conducted over approximately two years beginning in January 2021, 28 patients with advanced cholangiocarcinoma were treated with riboceranib combination therapy, administered either as a first-line or second-line treatment. The trial demonstrated a median overall survival (OS) of 12.8 months and a median progression-free survival (PFS) of 6.3 months, nearly double the typical 6- to 7-month survival period seen in patients with inoperable cholangiocarcinoma. Notably, among patients who received riboceranib combination therapy as first-line treatment, the ORR was 50.0%. HLB plans to review additional investigator-led clinical data from this study to further expand its pipeline. New drug R&D for cholangiocarcinoma is active globally China's TransThera Sciences is currently conducting a Phase 3 clinical trial in cholangiocarcinoma patients, with studies underway not only domestically but also in the United States, the United Kingdom, China, and other regions. Tinengotinib is a next-generation FGFR inhibitor designed for advanced cholangiocarcinoma patients harboring FGFR mutations who have prior treatment history. According to TransThera Sciences, this multi-kinase inhibitor features a unique FGFR binding mechanism that bypasses acquired resistance pathways. Clinical trial results showed that tinengotinib is effective in patients with advanced cholangiocarcinoma harboring FGFR mutations who have previously received systemic chemotherapy. In the clinical trial, patients were categorized into four groups based on their FGFR mutation status and treatment history. ▲Group A1 (13 patients): Patients with FGFR2 fusions whose disease progressed following treatment with conventional FGFR inhibitors ▲Group A2 (10 patients): Patients with FGFR2 fusions who initially responded to conventional FGFR inhibitors but subsequently relapsed ▲Group B (12 patients): Patients with non-fusion FGFR mutations ▲Group C (13 patients): Patients with no FGFR mutations (FGFRwt). Eisai has recently launched its FGFR2 inhibitor, Tasfygo, in Japan, boldly entering the cholangiocarcinoma market. In a Phase 2 study, Tasfygo achieved an ORR) of 30.2% (90% CI: 20.7–41.0), statistically surpassing the pre-set tumor response threshold of 15%. Meanwhile, U.S.-based Jazz Pharmaceuticals plans to launch its HER2-targeted therapy, 'Ziihera,' globally. Jazz Pharmaceuticals secured the development rights for Ziihera from U.S. biotech company Zymeworks in 2022 and has been conducting clinical studies. Accelerated approval was granted for this therapy in the U.S. last November, and it is now positioned to expand into international markets, including Korea. Its basis of approval HERIZON-BTC-01, Ziihera demonstrated an ORR of 52% with a duration of response (DOR) of 14.9 months.
- Company
- Obesity drug craze affects global pharma subsidiaries in KOR
- by Son, Hyung Min Apr 04, 2025 05:58am
- Whether or not a new obesity drug in the glucagon-like peptide (GLP-1) family has been launched has had a significant impact on the performance of Novo Nordisk and Lilly Korea. Last year, Novo Nordisk’s sales increased by 63% upon the launch of Wegovy in the domestic market. In the case of Lilly Korea, sales decreased slightly due to the delay in the launch of the licensed Mounjaro into the market in 2023 and the sluggish sales of some other products. According to the Financial Supervisory Service on the 3rd, Novo Nordisk Korea's sales increased 63% from KRW 230.2 billion in 2023 to KRW 374.7 billion last year. Operating profit increased 65% from KRW 8.3 billion to KRW 13.7 billion over the same period. Novo Nordisk’s sales growth was driven by Wegovy. According to the market research institution IQVIA, Wegovy recorded sales of KRW 60.3 billion in the same quarter since its launch in October last year. Wegovy, which was approved in Korea in April 2023, is a GLP-1 formulation composed of semaglutide, which has been confirmed to have effects on reducing weight and glycated hemoglobin. Novo Nordisk developed the obesity treatment drug Wegovy, a semaglutide that confirmed the weight loss effect of patients during the clinical trial of its GLP-1 class diabetes drug candidate and is administered once a week. Novo Nordisk and Lilly Korea Novo Nordisk’s insulin product also contributed to the growth in its sales. According to the market research institution UBIST, sales of its combination of liraglutide, a GLP-1 analogue, and the insulin degludec, Xultophy, reached KRW 15.1 billion last year, up 26% from the previous year. In addition, the once-weekly insulin product Tresiba and the insulin combination product Ryzodeg also recorded sales of KRW 38 billion and KRW 31.3 billion last year, up 3% and 7%, respectively. Novo Nordisk plans to further strengthen its position in the field of obesity. Currently, Novo Nordisk is conducting a global Phase III clinical trial of its new obesity drug, ‘CagriSema,' following the success of its previous drugs, Wegovy and Saxenda. CagriSema is a combination of 2.4 mg of semaglutide, the main ingredient of Wegovy, and 2.4 mg of the long-acting amylin analogue, Cagrilintide. This drug is regarded as a next-generation obesity drug. In clinical trials, CagriSema has been shown to be 23% more effective in weight loss than existing single-agent semaglutide. The expected end of clinical trials is in the first quarter of next year, after which Novo Nordisk plans to apply for approval from major regulatory agencies around the world. Lilly shows slow performance due to non-launch of Mounjaro On the other hand, the performance of Lilly Korea’s Mounjaro, which is also a GLP-1 class diabetes and obesity drug, showed a slight decline. Lilly Korea recorded sales of KRW 164.2 billion last year, down 2% from KRW 167.8 billion the previous year. Operating profit was KRW 10.3 billion, down 1% from 2023. In the case of Lilly Korea, it is analyzed that sales have not increased significantly due to the delay in the launch of Mounjaro. Mounjaro is a new diabetes drug developed by Lilly. Mounjaro acts on both the gastric inhibitory peptide (GIP) receptor and the GLP-1 receptor to promote insulin secretion, improve insulin resistance, and reduce glucagon secretion, thereby reducing blood sugar levels before and after meals. Obesity drugs Wegovy, Zepbound, Saxenda Mounjaro has the advantage of not only controlling blood sugar levels but also having an excellent weight loss effect. Mounjaro has proven its weight loss effect through the results of the Phase III SURMOUNT-1 clinical trial, which was conducted on overweight adult patients who are not diabetic, have a body mass index (BMI) of 30 kg/m2 or higher, or have one or more comorbidities, and who were administered Mounjaro once a week. Lilly launched the same-ingredient obesity treatment, Zepbound, in the US market in November 2023, as it has confirmed the weight loss effect in the clinical trial of the drug in the US. In Korea, the drug was approved as a treatment for diabetes in June 2023 and secured additional indications as a new obesity drug with the same product name in August last year. However, its launch in the domestic market has not yet taken place. In addition, sales of Lilly Korea’s Trulicity and Cymbalta were also sluggish last year. Sales of Trulicity, a GLP-1 class diabetes drug, fell 16% from KRW 44.4 billion the previous year to KRW 37.2 billion. Sales of Cymbalta, an antidepressant, fell 52% from 2023 to KRW 5.1 billion. Lilly Korea is looking forward to the success of the SGLT-2 inhibitor Jardiance. Jardiance's sales last year rose 14% year-on-year to KRW 66.3 billion. It benefited from the withdrawal of its rival product, Forxiga, from the market last year. In addition, the company is aiming for a rebound by launching Ebglyss, a new atopic dermatitis drug, in January this year. Lilly is also preparing a next-generation diabetes and obesity drug. Retatrutide, which is being developed by Lilly, is a next-generation diabetes and obesity drug that acts on three receptors: GLP-1, GIP, and GCG (glucagon). To date, no new obesity drugs have been commercialized using this mechanism. In the Phase II clinical trial, Retatrutide demonstrated a weight loss effect of 22.8% and 24.2% when administered at 8 mg and 12 mg at week 48, respectively. This is a higher weight loss effect than the 20.2% of the existing GLP-1 and GIP targeting Mounjaro. Currently, Lilly is confirming the potential of retatrutide not only in obesity but also in various chronic diseases such as diabetes and liver disease.
- Company
- New drug 'Niktimvo' for cGVHD receives ODD in Korea
- by Eo, Yun-Ho Apr 04, 2025 05:58am
- 'Niktimvo,' a new drug for the treatment of chronic graft-versus-host disease (cGVHD), has been designated as an orphan drug in South Korea. The Ministry of Food and Drug Safety (MFDS) announced this on the notifications of Orphan Drug Designation (ODD). Niktimvo (axatilimab)'s basis for ODD indication is for the treatment of 'adults and pediatric patients over 40 kg with chronic graft-versus-host disease (cGVHD) after failure of two or more systemic therapy.' In August last year, Niktimvo secured the U.S. Food and Drug Administration (FDA) approval It is a cGVHD treatment jointly developed by Incyte Corporation and Syndax Pharmaceuticals, a company in Massachusetts specializing in developing anticancer agents, through the exclusive agreement for global joint-development‧launching. GvHD is a serious complication that often occurs after allogeneic hematopoietic stem cell transplant (allo-SCT). Donor T cells from the transplanted graft recognize the recipient’s normal cells as foreign and attack them, affecting multiple organs, including the skin, gastrointestinal tract, liver, and lungs. Since symptoms can manifest throughout the body, GvHD causes additional challenges for patients who have survived allo-SCT, significantly impacting their quality of life. Corticosteroids are used as the first-line treatment; however, approximately 50% of patients fail to respond. In such cases, with no established standard therapy available, there has been an unmet need for effective treatment options. Regarding this, Niktimvo has emerged as a promising new therapeutic option with a novel mechanism of action to address the severe complications in chronic GVHD patients after previous treatments. In South Korea, Novartis Korea's 'Jakavi (ruxolitinib)' was listed on the reimbursement list in November 2023. Sanfi Korea's 'Rezurock (belumosudi)' is in the reimbursement process. The efficacy of Niktimvo was demonstrated through the AGAVE-201 study, which enrolled 241 children and adult patients with refractory and chronic GvHD who received two prior systemic therapies. Clinical results showed that the primary endpoint was met in every cohort treated with Niktimvo, with sustained responses observed across all organ systems and patient subgroups. Among patients receiving the approved dose of 0.3 mg/kg every two weeks, 75% achieved an objective response (ORR) within the first six months of treatment, with a median time to response of 1.5 months. Additionally, 60% of patients maintained their response after 12months of treatment.
- Policy
- COVID-19 Vaccine Damage Compensation Act passes NA
- by Lee, Jeong-Hwan Apr 04, 2025 05:58am
- A special law that compensates and supports patients who have suffered damage after COVID-19 vaccination was passed at the plenary session of the National Assembly on the afternoon of the 2nd. Also, a partial amendment to the Framework Act on Health and Medical Services, which includes the establishment of a Health and Medical Manforce Planning Estimation Committee under the direct control of the Minister of Health and Welfare, passed the plenary session along with the special act. The National Assembly passed the 'Special Act on Compensation for Damage Caused by COVID-19 Vaccination' with 263 in favor and 2 abstentions out of 265 members present. The Act on the Medical Manpower Planning Committee was passed at the plenary session with 247 in favor, 11 against, and 8 abstentions out of 266 members present. The Special Act expands the scope of vaccine damage compensation, such as by presuming that there is a causal relationship if the temporal correlation between COVID-19 vaccination and the disease occurrence is proven. The government has been administering vaccinations and providing state compensation in accordance with the current Infectious Disease Control and Prevention Act. However, the need for the Special Act was raised as there were indications that the damage caused by COVID-19 vaccination was not being properly compensated, due to limited causality of the damage being accepted. The Special Act, which passed the plenary session, was passed by the National Assembly Health and Welfare Committee in January with consensus from the ruling and opposition parties. The Medical Manpower Planning Committee Act, which will deliberate the number of medical school enrollees from 2027, has also passed the National Assembly, but it is unlikely to serve as a clue to resolve the conflict between the government and the medical community in Korea. The amendment proposes the establishment of the Medical Manpower Planning Committee, an independent deliberation body under the direct control of the MOHW, to deliberate on the estimation of medical personnel by job type. The committee members will consist of no more than 15 experts recommended by medical provider representative organizations, consumer representative organizations, and relevant academic societies, with the majority of the members recommended by providers, such as the Korean Medical Association (KMA), and the chairman of the committee will be elected from among the members recommended by the academic community. In addition, the revised act ensures the independence of the committee and ensures transparency by disclosing the minutes and reference materials, and the appointment of a Medical Manpower Planning center to ensure the professionalism of the planning work. When the committee estimates the size of the medical personnel required, the Health and Medical Services Policy Deliberation Committee, chaired by the Minister of MOHW, will then determine the number of medical school students.
- Company
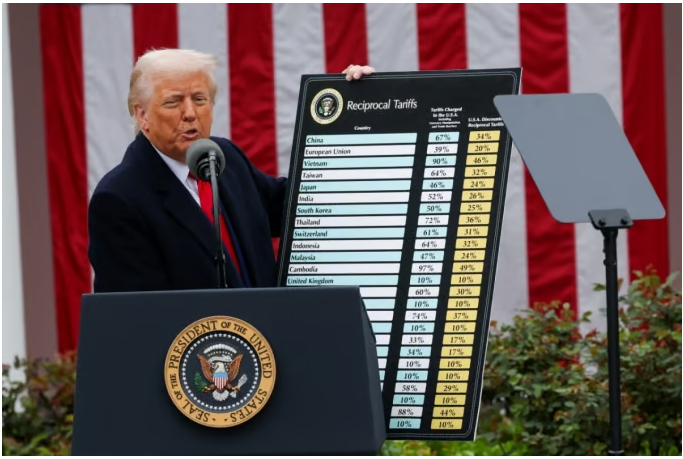
- US postpones 'Drug Tariffs', but industry eyes the situation
- by Kim, Jin-Gu Apr 04, 2025 05:57am
- The US Trump administration announced reciprocal tariffs against the entire world, but “postponed” the application of tariffs on pharmaceuticals, because it is “considering separate industry-specific tariffs” on semiconductors, key minerals, and pharmaceuticals. The domestic pharmaceutical and bio industry is relieved for now but is keeping a close eye on the White House's next move. As the U.S. has been the largest exporter of pharmaceuticals for 3 consecutive years, the specific tariff rates and items subject to the tariffs are expected to have a significant impact on the profits and losses of companies. US President Donald Trump announced on the 2nd (local time) that he would impose a 25% reciprocal tariff on South Korea. However, pharmaceuticals were excluded from this measure. The White House explained that it would impose separate tariffs on pharmaceuticals. The White House said in a separate briefing after President Trump's announcement of tariffs that “this measure does not apply to items already subject to tariffs such as automobiles, steel, aluminum, and lumber under Section 232 of the Trade Expansion Act.” It went on to explain that “President Trump is also considering separate industry-specific tariffs on semiconductors, pharmaceuticals, and key minerals, so these items are also not included in the reciprocal tariffs.” The domestic pharmaceutical and biopharmaceutical industry expressed relief for the time being because the companies have bought time to respond to the US drug tariff, at least temporarily. There are various ways for the pharmaceutical and biopharmaceutical industry to respond to the US drug tariff. In the short term, the method of moving stockpiles to the US in advance can be used. In the mid-to-long term, the industry is also considering producing products directly in the US or outsourcing manufacturing to US companies. Celltrion has secured sufficient inventories of biosimilars that can be procured locally until the third quarter of 2025. In addition, if tariffs are actually applied, the company plans to focus on exporting APIs, which are subject to lower tariffs, rather than finished drugs, which are subject to relatively higher tariffs. Furthermore, in the mid-to-long term, the company is also considering securing manufacturing facilities in the United States. SK Biopharmaceuticals, which sells the new epilepsy drug ‘Xcopri (cenobamate)’ in the United States, has a structure in which it manufactures active pharmaceutical ingredients in Korea, packs them in Canada, and then exports them to the United States. SK Biopharmaceuticals has been promoting this local production strategy in the United States for several years to stabilize its supply chain, and after transferring production technology and completing the verification procedures, it received approval from the US FDA in the second half of last year. Samsung Bioepis, which exports biosimilars to the US, is already cooperating with several contract manufacturing organizations (CMOs) overseas, so it is expected to be able to quickly adjust its strategy if a tariff is imposed. However, as the possibility of imposing tariffs on pharmaceuticals remains high, the pharmaceutical and biopharmaceutical industry is paying close attention to the specific tariff rates for pharmaceuticals and the items subject to tariffs. In this regard, President Trump announced at a press conference on the 18th of last month that the tariff on pharmaceuticals would be “25% or higher.” Some predict that basic and essential drugs will be exempted from tariffs. They note that public opinion in the United States is negative about imposing tariffs on basic and essential drugs. In the case of Huons, it exports lidocaine injections to the United States. This product is a basic drug that is in short supply in the United States and is said to have only one or two local manufacturers. So even if tariffs are imposed on pharmaceuticals, such basic drugs are expected to not be subject to them. The same is true for GC Biopharma. GC Biopharma exports the blood product Alyglo to the United States. Blood products are essential medicines in the United States, and there has been a shortage of supply in the country for several years. The company expects that blood products, including Alyglo, will not be subject to tariffs. In the United States, there is ongoing criticism that tariffs should not be imposed on basic and essential medicines. The Association for Accessible Medicines (AAM), a US generic drug lobby group, has expressed concern that if tariffs are imposed on medicines, the number of medicines in short supply, currently at 127, will surge to 215. The Kaiser Family Foundation, a US non-profit health organization, has warned that imposing tariffs on pharmaceuticals could cause the US healthcare spending growth rate to jump from the current 5% to as much as 60%. The United States has been Korea's largest pharmaceutical export destination for 3 consecutive years since 2022. Last year, pharmaceuticals exported from Korea to the United States amounted to USD 1.35809 billion (about KRW 2 trillion). This is an 18% increase compared to USD 903.3 million in 2023. However, the pharmaceutical trade balance with the United States has been in the red. The trade deficit with the United States was USD 1.1317 billion in 2022, USD 955.54 million in 2023, and USD 292.07 million last year.
- Company
- Qarziba may be prescribed in general hospitals in KOR
- by Eo, Yun-Ho Apr 03, 2025 05:55am
- Qarziba, an immunotherapy drug that targets neuroblastoma, may now be prescribed at general hospitals in Korea. According to industry sources, Recordati Korea's immunotherapy drug for high-risk, recurrent, and refractory neuroblastoma, Qarziba Inj (dinutuximab beta), has passed the drug committees (DCs) of the ‘Big 5 general hospitals’ in Korea, including Samsung Medical Center, Seoul National University Hospital, Asan Medical Center, and Severance Hospital. The company has been expanding the drug’s prescription in Korea since receiving reimbursement listing in December last year. As the first drug covered through the approval-evaluation-negotiation linkage system, the drug was approved only 13 months after it was approved in Korea. According to the Ministry of Health and Welfare notice, children from 12 months or older to less than 20 who have not received anti-GD2(disialoganglioside2) antibody treatment before with ▲high-risk neuroblastoma (INSS stage 4 disease or stage 2-3 and have MYCN gene amplification’ and have shown partial or better response to induction chemotherapy and have had hematopoietic stem cell transplant and initiated within 6 months of the transplant, and ▲recurrent or refractory neuroblastoma may receive Qarziba with reimbursement. The drug demonstrated its effect through major pivotal studies, including the Phase III APN311-302 trial. Main results showed that the 5-year event-free survival (EFS) rate for the Qarziba treatment group (378 patients) among high-risk neuroblastoma patients recieivng maintenance therapy was 57%, and the five-year overall survival (OS) rate was 64%, which was statistically significantly higher than that of the control group (466 patients) that did not receive Qarziba immunotherapy., which was 42% and 50%, respectively. Ji-won Lee, a professor of the Department of Pediatric Hematology-Oncology at Samsung Medical Center, said, “This disease has a particularly poor prognosis and a large disease burden, so it is very important to implement an appropriate maintenance therapy that can minimize minimal residual disease (MRD) to prevent recurrence when treating neuroblastoma. Qarziba has significantly improved the survival of patients with high-risk neuroblastoma as maintenance therapy compared to those that did not receive maintenance immunotherapy, shifting the treatment paradigm.” “Major overseas guidelines, such as the International Society of Pediatric Oncology Europe Neuroblastoma (SIOPEN) and the National Comprehensive Cancer Network (NCCN), have already recommended anti-GD2 antibodies such as Qarziba as standard maintenance immunotherapy for high-risk neuroblastoma,” said Jung-yoon Choi, Professor of the Department of Pediatrics at Seoul National University Children's Hospital. ”With the Korean patients also able to receive the global standard of care, I believe this will change the treatment environment in Korea.”
- Company
- "COVID-19 differs from influenza…vaccination essential"
- by Whang, byung-woo Apr 03, 2025 05:55am
- "COVID-19 can lead to 'long COVID,' and its progression tends to be more severe among the elderly and immunocompromised individuals. It is crucial to emphasize COVID-19 prevention as the saying goes, 'an ounce of prevention is worth a pound of cure.' After the announcement of the endemic in 2023, COVID-19 has repeatedly mutated, thus regarded as becoming a 'resident virus' that makes a comeback similar to influenza. Although determining the exact seasonal pattern of COVID-19 may require more time, the government provides vaccination to high-risk groups, such as individuals aged 65 and older. Professor Woo Joo Kim in the Department of Infectious Diseases at Korea University Guro HospitalDuring a meeting with Daily Pharm, Dr. Woo Joo Kim, a professor in the Department of Infectious Diseases at Korea University Guro Hospital, emphasized the importance of reducing the disease burden by putting efforts into increasing the COVID-19 vaccination rate. Currently, the legal status of COVID-19 has been designated as a class 4 infectious disease in South Korea after the endemic announcement in 2023. Like influenza, COVID-19 is categorized as an infectious disease requiring daily monitoring. Despite the initial expectation that COVID-19 will show seasonal spikes, it has not demonstrated a clear seasonal pattern like influenza. Dr. Kim explained, "COVID-19 is likely in a 'transition period.' The severity is lowered compared to the earlier phase of the pandemic due to vaccination, but it is yet to be defined as a seasonal virus," adding, "In the past few years, COVID-19 made a comeback in around January-February and around August. However, it is yet too soon to generalize." "Over the past four years, the severity of COVID-19 has diminished compared to its early phase, thanks to immunity acquired through vaccination and natural infection. However, high-risk groups, including the elderly, individuals with underlying conditions, immunocompromised patients, pregnant women, and residents of long-term care facilities, continue to face a significant burden of disease, with a high risk of severe illness or death," Dr. Kim said. Dr. Kim's attention to the COVID-19 vaccine during this transitional period is because COVID-19 exhibits infection characteristics distinct from those of influenza. While both diseases may be perceived as similar, there are clear differences in infection patterns, causative pathogens, and complications. The influenza virus causes influenza, whereas COVID-19 is caused by SARS-CoV-2. "The COVID-19 virus not only targets the respiratory system but also invades the vascular system, kidneys, and other organs, causing a systemic infection with higher severity and fatality rates," Dr. Kim stated. "Long COVID, affecting the entire body as a sequela, can occur, and its impact tends to be more severe in older individuals or those with weakened immune systems." Although COVID-19 and influenza share similar symptoms and some complications, which can lead to them being conflated, experts warn that this may result in the risks of COVID-19 being underestimated, calling for heightened vigilance." For these reasons, the government is currently administering COVID-19 vaccines to high-risk groups, including individuals aged 65 and older, immunocompromised persons aged at least 6 months, and residents of facilities vulnerable to infections, to prevent severe illness and death. The ongoing issue is whether to include the COVID-19 vaccine in the National Immunization Program (NIP). There is a growing consensus that incorporating the COVID-19 vaccine into the NIP is essential to boost vaccination rates for the currently administered vaccines." Dr. Kim said, "In addition to preventing infections, hospitalizations, and deaths at the individual level, incorporating the COVID-19 vaccine into the NIP is essential for sustaining our national population. For instance, by systematically benchmarking the well-established NIP for the influenza vaccine in individuals 65 and older, we can effectively plan and implement a similar strategy for the COVID-19 vaccine." There is ongoing concern about determining the optimal vaccination schedule, as it remains difficult to predict the precise timing of the COVID-19 surge. Dr. Kim emphasized, "When establishing the vaccination schedule for the COVID-19 vaccine, it is crucial to base decisions on scientific evidence regarding the outbreak period, the duration of antibody persistence, and the vaccine's ability to counter emerging variants." He advised, "Antibodies may not form adequately or may quickly be depleted in individuals with severe immunocompromise. It is advisable to tailor the dosing frequency and timing based on each patient's immune status." Dr. Kim added, "High-risk groups such as immunocompromised individuals and residents of long-term care facilities face a significantly higher risk of severe illness if infected, yet their vaccination rates remain low. If we were to improve COVID-19 vaccination rates, government's promotional activities is essential." "COVID-10 vaccination can be compared to wearing a seat belt, lowering severity" Currently, two mRNA COVID-19 vaccines are available in Korea. Aside from a minor difference in antigen dosage (approximately 30 to 50 micrograms) their basic compositions, including encapsulation in lipid nanoparticles, are virtually identical. In clinical practice, healthcare providers typically use whichever vaccine is distributed to their institutions and available for immediate use. However, due to differences in domestically imported volumes, over 80% of vaccinations administered during 2023–2024 were Pfizer's Comirnaty. In global Phase 3 trials, Comirnaty demonstrated a 95% preventive efficacy across COVID-19–naïve and previously infected participants, with overall safety profiles remaining favorable. Dr. Kim explained, "mRNA vaccines like Comirnaty induce a robust T-cell immune response, ensuring that the benefits of preventing severe disease and death are sustained over a long period. The Korea Disease Control and Prevention Agency also strongly emphasizes these outcomes when recommending COVID-19 vaccination." One of the concerns raised about COVID-19 vaccines has been safety issues that emerged during the pandemic. However, Dr. Kim believes that confidence in the vaccine's safety has increased. He noted, "Among high-risk groups such as those aged 65 and older or individuals with underlying conditions, no significant adverse reactions have been observed, and the benefits of vaccination are substantially greater," adding, "With sufficient vaccination experience now accumulated and those who experienced adverse reactions subsequently excluded from further dosing, the incidence of adverse events in recent vaccination cohorts is very low." He particularly compared vaccination to wearing a 'seat belt.' Although wearing a seat belt can be uncomfortable and does not guarantee 100% prevention of injuries, it significantly reduces the risk when an accident occurs. Similarly, COVID-19 vaccination is essential to lower the risks of infection and severe disease. Lastly, Dr. Kim stressed that a coordinated effort at the individual, societal, and government levels remains critical for effective COVID-19 management. Dr. Kim said, "High-risk groups, such as the elderly and those with underlying conditions, must recognize their risk of infection and proactively get vaccinated, and if they experience symptoms, they should promptly seek diagnosis and treatment," adding, "I believe including the COVID-19 vaccine in the NIP can be expedited if individuals actively voice their concerns." "On a societal level, we need to foster a 'take a break when you're sick' culture, and at the government level, it is essential to establish an independent monitoring system solely for COVID-19," Dr. Kim added. "The government must quickly set vaccination schedules and develop promotional strategies based on up-to-date data on the latest COVID-19 outbreak patterns, disease burden, and the efficacy and safety of vaccines."
- Opinion
- [Reporter's View] Preparations for US pharmaceutical tariff
- by Kim, Jin-Gu Apr 03, 2025 05:55am
- US President Donald Trump designated April 2 (local time) as 'America's Day of Liberation.' The day the US will announce reciprocal tariff rates for various countries. US reports expect details on tariff-affected countries, rates, and applicable product categories to be disclosed as early as the evening of the 1st. The specific tariff rates will be known in South Korea by the morning of the 2nd or, at the latest, the morning of the 3rd in Korean time. The United States is Korea's largest market for pharmaceutical exports. Last year, Korean pharmaceutical exports to the US reached US$1.359 billion (approximately KRW 2 trillion), a 50% increase compared to 2023. When expanded to include the broader market, exports have surged from US$33 million a decade ago in 2014 to over 40 times that amount. The US accounted for 18% of Korea's total pharmaceutical export revenue last year, making it the largest single market among all countries. This fact underscores why US tariffs on pharmaceuticals could have a significant impact. Industry attention is now focused on whether Korean pharmaceuticals will also be subject to tariffs, and if so, which specific drugs and at what rates. A 25% tariff rate is the most likely scenario. During a press conference on March 18, President Trump even hinted that pharmaceutical tariffs 'could be 25% or more.' However, it remains unclear whether the forthcoming reciprocal tariff announcement scheduled for April 2 will include pharmaceuticals, or if tariffs on pharmaceuticals will be imposed separately. Some analysts predict that pharmaceuticals might be excluded from the tariff measures, arguing that imposing tariffs on them would likely lead to a surge in domestic healthcare costs. Indeed, recent projections indicate that the number of drugs experiencing supply shortages in the US could exceed 215 (up from 127), and US generic drug prices may increase by an average of 18%. One report even warned that US healthcare spending could skyrocket from a baseline of 5% to as high as 60%. Another opinion suggests that tariffs could be applied only selectively, targeting certain countries with large trade deficits, such as some European nations, or excluding critical categories like essential or supply-shortage drugs. Nonetheless, prevailing forecasts still lean toward the imposition of pharmaceutical tariffs. Should tariffs be levied on domestically produced drugs, it could deliver a severe hit to Korea's pharmaceutical and biotech industries. Companies are left with few options and must concentrate on creating exit strategies. The Trump administration has reiterated a 'tariff first, negotiate later' approach. Even if tariffs are eventually imposed on Korean pharmaceuticals, there remains potential to mitigate the impact through subsequent negotiations. Despite South Korea being identified as a 'notorious trade imbalanced country,' industry negotiators argue that it is important to emphasize that Korea has consistently run a trade deficit with the US in the pharmaceutical sector. In the medium to long term plan, South Korea must also accelerate reforms in its drug pricing and innovative pharmaceutical company certification systems. The United States Trade Representative (USTR) has previously released a report stating that Korea's drug pricing policies and innovative pharmaceutical company certification system disadvantage foreign companies. Both systems are currently undergoing revisions, and if given the opportunity to negotiate with the US, South Korea should actively highlight these improvements at the bargaining table.
- Company
- J&J MedTech launches PFA Varipulse platform
- by Whang, byung-woo Apr 03, 2025 05:54am
- Pic of Varipulse Johnson & Johnson MedTech announced on the 1st that it will launch the Varipulse platform, an arrhythmia treatment solution that uses three-dimensional pulsed field ablation (PFA), in Korea. As the most common arrhythmia in the world, Atrial fibrillation (AFib) is a condition in which the atria beat irregularly and rapidly. In particular, the prevalence of atrial fibrillation in Korea is on the rise with the aging population. According to the Korean Heart Rhythm Society, the incidence of this disease has increased by about 1.5 times in the last 10 years, and about 1 million people are suffering from atrial fibrillation in Korea. The Varipulse platform is the first 3D pulsed field ablation treatment solution in Korea developed for the treatment of drug-refractory paroxysmal atrial fibrillation. PFA has recently gained attention as a treatment for atrial fibrillation and is an innovative treatment that reduces the side effects of conventional radiofrequency ablation and cryoballoon ablation. PFA uses an electric field (pulse field) to selectively remove lesion tissue that causes arrhythmia, minimizing damage to surrounding normal tissue and shortening procedure time and patient recovery time. Currently, Varipulse is the only PFA solution introduced in Korea that has been developed based on a three-dimensional cardiac mapping (3D mapping) system. The technology creates a precise three-dimensional image of the anatomical structure of the left atrium and allows the catheter to be accurately tracked in real-time. The size of the Varipulse Catheter can be adjusted to fit each patient's heart anatomy, allowing for an optimized procedure fit for each patient's heart shape. Also, the Varipulse platform can minimize patient radiation exposure by integrating a 3D cardiac structure mapping function with intracardiac echocardiography (ICE) to provide real-time images of the heart during PFA therapy. These innovative features are expected to shorten procedure time and significantly improve patient treatment outcomes. “We are pleased to be able to hasten the introduction of the innovative PFA treatment with Varipulse to many atrial fibrillation patients in Korea, following in the United States, Europe, Japan, China, and Canada. We expect that the launch of Varipulse will contribute to the improvement of atrial fibrillation patients and treatment in Korea,” said Jinyong Oh, Managing Director of Johnson & Johnson MedTech's North Asia region Oh added, “We will continue to work on fostering a patient-centric treatment environment and introduce innovative solutions to contribute to the development of Korea’s medical field.” Meanwhile, Johnson & Johnson MedTech established a medical technique education center for the treatment of cardiac arrhythmia at its Yongsan headquarters in Seoul last year, providing education and training to healthcare professionals on procedures related to the treatment of arrhythmia.
- Company
- ‘Leclaza combo will become the sole standard of care’
- by Son, Hyung Min Apr 03, 2025 05:54am
- Professor Byoung-chul Cho of the Department of Oncology at Yonsei Cancer Center “The Leclaza + Rybrevant combination therapy has shown a clear improvement in survival compared to Tagrisso monotherapy in the MARIPOSA trial. I am confident that this combination therapy will become the sole standard treatment for EGFR-positive non-small cell lung cancer in the future.” On the 31st, Professor Byoung-chul Cho of the Department of Oncology at Yonsei Cancer Center (Director of the Lung Cancer Center) held a briefing session on the MARIPOSA trial at Kiwoom Securities in Yeouido to evaluate the clinical trial results. The MARIPOSA trial compares the efficacy and safety of the combination of Leclaza and Rybrevant with the single-agent treatment using Targrisso, which is currently used as the first-line treatment for EGFR-positive non-small cell lung cancer. The final overall survival (OS) results of this study were unveiled at the European Lung Cancer Conference (ELCC 2025), which was held in Paris, France for 4 days starting on the 26th of this month. Leclaza is a new EGFR-positive non-small cell lung cancer drug developed by Yuhan Corp and is a third-generation tyrosine kinase inhibitor (TKI) that targets exon 19 and exon 21 (L858R). Janssen's parent company Johnson & Johnson has secured global rights to Leclaza and has been conducting a clinical trial of a combination therapy using Rybrevant, a targeted treatment option targeting the exon 20 and MET mutations. The results presented at this year's ELCC 2025 are the final OS analysis. OS is one of the important indicators for determining the clinical value of anticancer drugs. OS refers to the overall survival period from the start of treatment to the time of death. OS includes not only the side effects and complications of the treatment but also patients who died from causes other than cancer. Phase III MARIPOSA Study Design The clinical trial was conducted to compare the efficacy and safety of the Leclaza+Rybrevant combination with Tagrisso monotherapy in 1,074 patients with non-small cell lung cancer with the Exon19 and L858R mutations who had no previous treatment experience. Leclaza monotherapy was included to evaluate the contribution component of Rybrevant. Patients were randomly assigned to the Leclaza + Rybrevant group (429 patients), the Tagrisso group (429 patients), and the Leclaza group (216 patients) in a 2:2:1 ratio. After tracking patients for a median of 37.8 months, the Leclaza+Rybrevant group showed a statistically significant improvement in survival compared to the Tagrisso group (p-value