- LOGIN
- MemberShip
- 2025-12-22 16:49:58
- Company
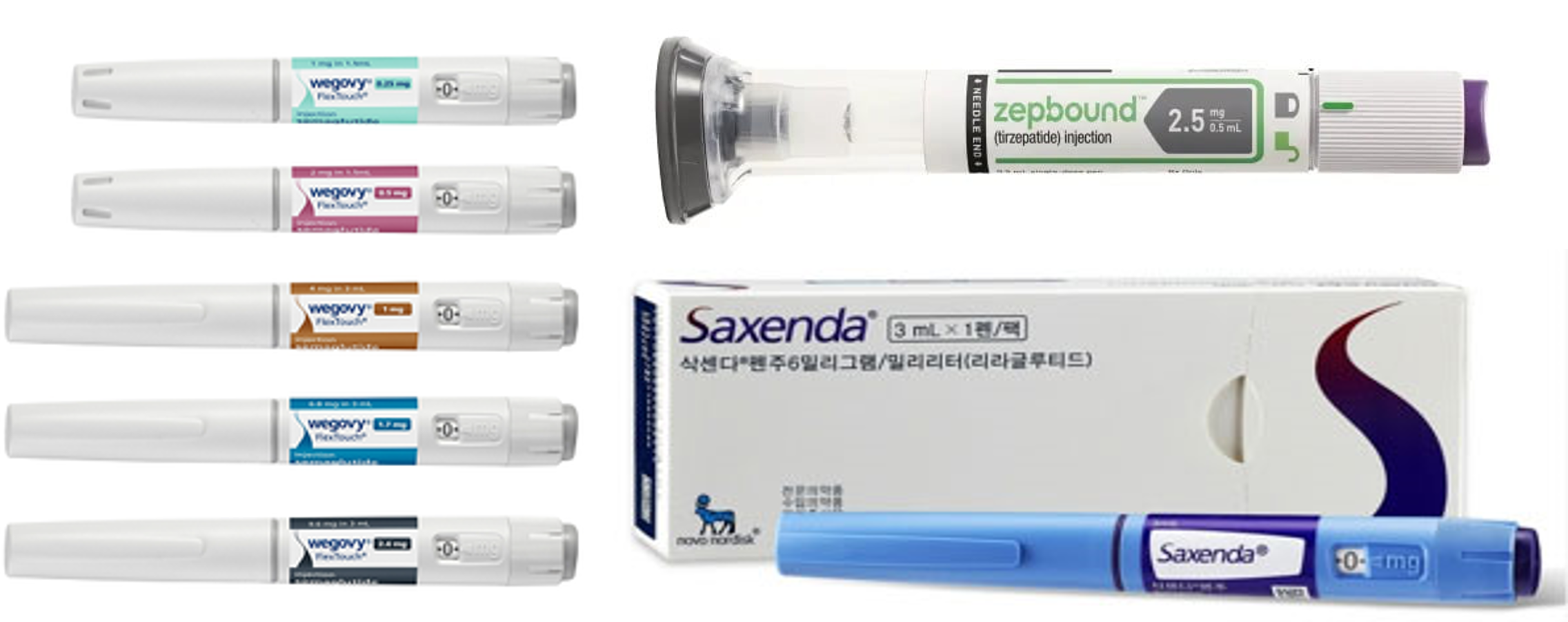
- Wegovy dominates the obesity drug market
- by Chon, Seung-Hyun Mar 13, 2025 05:59am
- The introduction of Novo Nordisk's Wegovy has shaken the market substantially. Since its launch in Q4, Wegovy has captured 60% of the entire market. Given the introduction of Wegovy, the obesity market expanded to the largest in history. It also consumed the market for Saxenda, containing the same class of active ingredients as Weogvy. It is unclear whether such growth will continue because of restricted non-face-to-face medical prescriptions of obesity drugs. However, the market continues to shake up whenever effective and safe new obesity drugs from multinational companies are launched. Record sales in 2024 obesity drug market…Wegovy's Q4 sales amounted to KRW 60.3 billion, with 64% market share According to pharmaceutical market research firm IQVIA, on March 10, last year's obesity drug market amounted to KRW 236.3 billion, up 32.8% compared to the previous year. The obesity drug market continued to set the largest size for seven consecutive years since 2018, exceeding KRW 200 billion for the first time in history. Last year's growth of the obesity drug market was led by Novo Nordisk's Wegovy. Wegovy launched in October last year and recorded KRW 60.3 billion in three months. Quarterly sales figures for key products in the obesity drug market. (Legend from left) Wegovy, Qsymia, Saxenda, obesity drug market (unit: KRW 100 million, source: IQVIA) Wegovy received approval from the Ministry of Food and Drug Safety (MFDS) in April 2023. It is a GLP-1-containing semaglutide that has been shown to reduce glycated hemoglobin. While conducting clinical trials for GLP-1 diabetes treatment candidates, Novo Nordisk confirmed the effects of lowering patient body weight and developed semaglutide-containing Wegovy as an obesity drug with a once-weekly formulation. Last year's Q4 obesity market size amounted to KRW 93.8 billion, an expansion of 154.5% Year-over-Year (YoY). Wegovy accounted for 64.4% of the entire obesity drug market. Wegovy is trending globally with its outstanding weight loss effects. Wegovy recorded last year's sales of NOK 58.2 billion (About KRW 11.7 trillion), an increase of 85.7% compared to 2023 sales of NOK 31.3 billion. Since its launch in the United States, the drug has been sold out with a rapid increase in demand. Before its official launch, Wegovy was already regarded as a secret weight loss solution among celebrities, including Tesla CEO Elon Musk, and there was a global shortage. Despite being priced KRW 500,000 higher than the other drugs, Wegovy generated significant interest following its domestic launch and was in short supply. Due to the introduction of Wegovy, the sales of Saxenda and Qsymia, which previously dominated the obesity drug market, have significantly decreased. Last year, Saxenda recorded sales of KRW 65.6 billion, down 1.7% from the previous year. The decrease in sales of Saxenda was seen three years after 2021. Saxenda reported a significant decline in sales in Q4 of last year. Saxenda's sales in Q4 of last year amounted to KRW 7.3 billion, down 27.3% YoY. The sales declined by 78.9% in a quarter from KRW 18.9 billion in Q3 of last year. Analysis suggests Wegovy, a GLP-1 drug similar to Saxenda, has captured the market for Saxenda. However, Qsymia's sales have not changed much since the launch of Wegovy. Qsymia recorded sales of KRW 39.1 billion last year, up 10.1% from the previous year. In Q4 last year, when Wegovy launched, Qsymia recorded sales of KRW 9.3 billion, down 7.1% from the previous year. However, it is unclear whether Wegovy will show marked growth this year. Previously, Wegovy was prescribed through non-face-to-face medical sessions. As concerns have been raised regarding unrestricted prescription of Wegovy through non-face-to-face medical sessions regardless of weight or obesity status, the health authority discontinued non-face-to-face prescription of obesity drugs as of December 16, 2024. The obestiy drug market fluctuates whenever new drugs launch…Saxenda has led the market for the past five years The obesity drug market underwent restructuring whenever promising new products are launched. Products containing sibutramine, which inhibits appetite, sold the most and once dominated the market. However, it has no longer been in sale due to the risk of cardiovascular side effects since 2010. The Korean market of obesity drug market was sluggish for a long time. Once worth a market size of KRW 116.2 billion in 2009, it dropped to KRW 66.7 billion over five years. Since 2015, the introduction of new products has led to a rebound in the market. In February 2015, Ildong Pharmaceutical obtained domestic approval for 'Belviq,' which the company acquired from the U.S.-based Arena Pharmaceuticals and led the recovery of the entire market. Belviq selectively works on the neurotransmitter serotonin receptor, which regulates appetite and emotion, suppressing appetite and increasing meal-related satiety. It has gained attention for being the new drug approved for weight-loss medication by the U.S. Food and Drug Administration (FDA) in 13 years. Kwang-dong Pharm has contributed to the market expansion after launching 'Contrave' in 2016. Kwang-dong Pharm acquired Contrave from the U.S.-based biotech company Orexigen Therapeutics. Contrave was approved by the European Medicines Agency (EMA) in 2015, and it is used to manage the weight of adults who are overweight or obese. After the launching of Belviq and Contrave, the obesity drug market expanded to KRW 92.8 billion and KRW 96.8 billion in 2017 and 2018, respectively. Yearly obesity drug market size (unit: KRW 100 million, source: IQVIA). Belviq, Saxenda, and Wegovy were launched in 2015, 2019, and 2024, respectively. Saxenda, launched in Korea in 2018, is the first glucagon-like GLP-1 agonist medication for obesity. It contains the same ingredient as Victoza (ingredient: liraglutide), which is prescribed to patients with type 2 diabetes but with different methods of administration and dosages. Saxenda became the top-selling drug in the market after recording sales of KRW 42.6 billion in 2019, just after its launch, and maintained the place for five consecutive years until 2023. Saxenda recorded sales of KRW 66.8 billion in 2023. Saxenda took up a 37.5% market share of the obesity market in 2023. The obesity drug market set a record in 2019 with KRW 134.1 billion in 10 years. In 2023, it recorded KRW 178 billion, setting a record for five consecutive years. Qsymia, marketed by Alvogen Korea, has also contributed to the expansion of the obesity drug market. Launched in late 2019, Qsymia is a combination therapy containing 'phentermine' and 'topiramate.' Alvogen Korea secured domestic sales rights from the U.S.-based VIVUS in 2017. In late 2019, Alvogen Korea signed a co-promotion agreement with Chong Kun Dang to begin full-scale domestic sales. Qsymia recorded sales of KRW 35.5 billion in 2023. The sales of Qsymia were comparable to Saxenda. Despite oral administration, Qsymia has a relatively small amount of psychotropic drug ingredients, and it has the advantage that it can be prescribed for an extended period. Alvogen Korea's extensive sales networks in the domestic obesity market, gained from its previous experience selling Furing and Furimin, synergized with Chong Kun Dang's business power to penetrate the market with Qsymia rapidly. With the launch of Wegovy last year, the obesity drug market underwent another restructuring, and the introduction of Mounjaro and other next-generation obesity treatments is expected to reshape the landscape further. Eli Lilly’s Mounjaro received approval from the MFDS in June 2023. Mounjaro, a once-weekly injectable, is a next-generation GLP-1 analog that activates GLP-1 and GIP receptors. It has been demonstrated that Mounjaro has superior weight loss compared to Wegovy.
- Company
- "High hopes for Blincyto as consolidation therapy for ALL"
- by Whang, byung-woo Mar 13, 2025 05:58am
- Blincyto (blinatumomab), a treatment for acute lymphoblastic leukemia (ALL), is approved for an expanded indication as a consolidation therapy. Its role in clinical settings will be broadened. Experts view that having more treatment options is favorable, as the consolidation therapy option has been limited following the first-line treatment of B-precursor ALL until now. Dr. Jae-Ho Yoon, Professor in the Department of Hematology at Seoul St. MaryOn March 12, Amgen hosted a press conference celebrating the approval of an expanded indication for Blincyto as a consolidation therapy to treat B-precursor ALL. The company highlighted the potential role of the drug. On the 14th of last month, the Ministry of Food and Drug Safety (MFDS) granted approval of indication for Blincyto as a consolidation therapy for Philadelphia chromosome-negative (Ph-negative) B-precursor ALL. The approval broadened the treatment area of the drug from treating adults with relapsed or refractory B-cell precursor ALL with minimal residual disease (MRD) to treating adults of young children with Ph- B-cell precursor ALL as a consolidation therapy up to Stage 4. Patients with Ph- B-cell precursor ALL are known to relapse often despite reaching MRD negative through remission induction therapy using existing chemotherapy. Patients with ALL may experience difficulties in long-term survival despite undergoing stem cell transplantation. Therefore, ALL still has high unmet needs. Approval of a new indication for Blincyto as a consolidation therapy for patients who already had induction therapy using existing chemotherapy has gained attention since it was based on clinical data demonstrating statistically significant effects on patients diagnosed early or who relapsed. According to the E1910 clinical study results, administering Blincyto as the first-line consolidation therapy after chemotherapy to adult patients (n=112) with B-precursor cell ALL who had MRD-negative remission (
- Policy
- Mandatory reporting of market supply suspended drugs
- by Lee, Hye-Kyung Mar 13, 2025 05:58am
- The mandatory reporting is being considered to include drug products of suspended production·importation and supply, and 'supply shortage drugs' of which supply has been halted for more than 1 month. The Ministry of Food and Drug Safety (MFDS; Minister, Yu-Kyoung Oh) stated that it would announce on March 11 an administrative notice of revision to the 'Regulation on Notification of Production·Importation·Supply suspended drug products' and will receive opinions until March 31. The regulation concerns the notification criteria for pharmaceutical companies reporting a supply shortage of drug products. The revision details include reducing the time for reporting the discontinuance of production, importation, and supply of drug products from 60 days in advance to 180 days in advance, and it establishes a new duty to report on production and importation shortage. The MFDS has established criteria for production·importation shortage to address the issue of lack of definition of drugs sold out and drugs in supply shortage. Revision (draft) to the According to the MFDS' criteria for reporting drug supply shortages due to reduced production·importation, two types have been set as ▲A decrease in production·import volume to less than half of the three-year annual average over the next one year ▲A decrease in supply or supply suspension, including a temporary halt in production·imports for at least three months or a market supply suspension for more than one month. In the case of supply reduction, if production or imports are expected to decrease to 50% or less of the three-year annual average for the upcoming one year as of the end of each quarter, a report must be submitted to the MFDS within one month of the quarter's end. Suppose production or imports are expected to be suspended for more than three months, and market supply is expected to be halted for over one month. In that case, a supply reduction plan must be established and reported within one month. However, if the temporary suspension of production·imports·supply is extended and meets the reporting criteria, the revised plan must be reported within 10 days of the change. Supply reduction and temporary supply suspension are excluded from reporting requirements in cases where ▲Temporary suspension or reduction of production·import occurs before the designation of a reportable item ▲Temporary suspension or reduction occurs naturally due to decreased market demand, or only specific packaging units are affected without significant disruption to the overall drug supply ▲Manufacturing·import·sales suspensions resulting from administrative sanctions. Additionally, reporting on temporary supply suspensions is exempt ▲If the product has been on the market for less than three years after approval or has been produced·imported two or fewer times in the past three years ▲If the company holds inventory equivalent to its quarterly production or import volume for the past year and faces no difficulty in market supply. Additionally, as previously announced by the MFDS, the revised regulations now require companies to report supply discontinuation 180 days in advance, moving up the reporting timeline from the previous 60 days. For permanent·temporary discontinuation of drug production·import·supply due to reasons such as withdrawal of product approval or contract termination, companies must report at least 180 days before the scheduled discontinuation date. Along with enhanced reporting duty, administrative penalties have also been revised. If a company reports supply discontinuation between 180 days before the discontinuation date and the actual discontinuation date, the first violation will result in a 7-day suspension of manufacturing operations. A second violation will lead to a 15-day suspension, a third violation will result in a 1-month suspension, and a fourth violation will incur a 3-month suspension of manufacturing operations. Failure to report production reduction will also result in penalties. A first violation will result in a warning, a second violation will lead to a 15-day suspension, a third violation will result in a 1-month suspension, and a fourth violation will lead to a 3-month suspension of manufacturing operations.
- Company
- ‘Keytruda’s 10-year milestones show its core value’
- by Whang, byung-woo Mar 13, 2025 05:58am
- Although many drugs have left their mark on the domestic pharmaceutical market, it is difficult to talk about the last decade without mentioning the immuno-oncology drug, Keytruda (pembrolizumab). As a drug that holds the title of the No. 1 in global sales, it has left various milestones in Korea, from sales to approved indications. This year marks the 10th anniversary of Keytruda’s approval in Korea, and the company is gearing up to drive another decade. The fact that Keytruda has recently been approved for a number of indications by the Cancer Disease Deliberation Committee is one of the reasons why the company's progress is drawing attention. The Oncology Business Unit of MSD Korea has been delivering its sincerity with the mindset that “cancer treatment is KEY TRUE DA (Key to the truth).” Dailypharm met with Min-kyung Kim, Associate Director (oversees lung cancer, gastrointestinal cancer, head and neck cancer, etc), and Joo-hyun Shin Associate Director (oversees breast cancer, gynecological cancer, urinary cancer, etc) of the Oncology Business Unit at MSD Korea to hear about the company’s blockbuster treatment, Keytruda. Keytruda’s 10-year approval holds title for being the immuno-oncology drug with the most indications in Korea As of February 2025, Keytruda is the most widely prescribed immuno-oncology drug in Korea indicated for 34 indications in 18 cancer types. In addition to the 2 directors who act as managers for the many indications, a total of 11 product managers (PMs) are in charge of Keytruda at MSD Korea. Specifically, under Director Kim, there are a total of 5 PMs for lung cancer, stomach cancer, biliary tract cancer, and head and neck cancer, and under Director Shin, there are a total of 6 PMs for breast cancer, gynecological cancer, and urinary cancer. Keytruda is regarded to have changed the paradigm of cancer treatment. How do the two directors feel about this? Min-Kyung Kim, Associate Director of the Oncology Business Unit at MSD Korea “Immunotherapy drugs are more effective when used early in the treatment process, when the patient's condition is good, and when used at the front line of treatment, they can bring the patients a step closer to a complete cure than before,” said Shin. “I am proud to be part of a team that is leading this change in the treatment paradigm and saving many lives.” Kim added, “I have been working at MSD for about 19 years, and I felt that all the team members are elite members who work with a great sense of mission because Keytruda is a treatment that is closely related to life. I joined the team in 2021, and I found that the team members are even more committed than they appear, and I think this is the driving force behind the growth of Keytruda over the past decade.” However, due to the vast indications for Keytruda, it is positive that there is a PM for each indication, but conversely, there may be concerns about a lack of communication or synergy between the PMs. This means that there may be concerns about the size of sales and team management. In response, the two directors emphasized that, although the role of each indication is important, a long-term strategy matters most due to the nature of Keytruda. “As the No. 1 drug, the company has organized the team with passionate and proactive PMs for the drug that collects industry-wide attention,” said Kim. “Sales are important, but we need to adjust our focus and efforts depending on new indications and momentum, and are trying to operate with clear standards while thinking about the results that can be achieved over the long term.” “The indications that are reimbursed and non-reimbursed, and the cancer types that have synergistic effects and those that do not, are creating a market or producing results,” said Shin. ”As a leader, we are constantly looking to derive new results through a process of constant feedback to see if there is anything we can do better or if there are any points to consider further.” The dilemma of expanding a treatment’s indications is 'Competition'... differentiation and market exploitation Although Keytruda has made great strides in the market over the past decade, its many indications for various types of cancers are also one of the tasks that must be resolved to compete with other treatments. Joo-hyun Shin, Associate Director of the Oncology Business Unit at MSD Korea On this, the two directors each brought up the topics of differentiation and market development, in consideration of the teams they are in charge of. So far, Keytruda has been the leading brand that has changed the paradigm of the cancer treatment environment, and has been promoting the keywords “first” and “only.” However, as these messages are gradually losing power, the company believes that it needs to promote other keywords. “Competition can also be interpreted as the threshold for immuno-oncology treatments has been lowered, which is certainly good news in this respect,” said Kim. ”However, the marketing team has a clear goal of gaining an edge over the competition, so we are focusing most on how to communicate the differentiated value of Keytruda (compared to competing products).” In addition, Shin emphasized, “From a marketing perspective, competition is not simply a matter of dividing up the market pie, but also about growing the overall size of the market, so it may be a little difficult, but we believe that it is the role of the marketing team to pioneer new markets.” There are also positive factors in the competitive situation in which Keytruda is placed. This is because it has achieved some results after applying for reimbursement for a total of 17 indications in 2023, after the 13 indications it applied for reimbursement in 2023. The Health Insurance Review and Assessment Service (HIRA) established reimbursement standards for 11 indications at the 1st Cancer Disease Review Committee (CDRC) in 2025 after 6 reimbursement attempts. The indications that passed were also diverse, including stomach cancer, esophageal cancer, endometrial cancer, rectal cancer, squamous cell carcinoma, cervical cancer, breast cancer, small intestine cancer, and biliary tract cancer. Although there are still many processes to go through, such as the Drug Reimbursement Evaluation Committee (DREC) and the National Health Insurance Service (NHIS) drug pricing negotiations, the fact that the drug passed the Cancer Disease Deliberation Committee review holds significance, as it was regarded as a reckless challenge at the time. “We took on the challenge literally recklessly with the idea that ‘Keytruda is the only option,’ to improve access to treatment for all cancer types, and I think this was possible because we all worked together with the common goal of improving patient access,” said Kim. “I feel like we're just getting started. “I think that, as Keytruda has done so far, the remaining procedures should be carried out one by one to pave the path,“ said Shin. MSD Korea’s Oncology BU Product Managers ”Keytruda’s approach to the next decade aims to expand accessibility" The two directors intuitively expressed that Keytruda is the drug that can bring about ”more tomorrows” to patients. In addition to benefiting patients, this is also the goal of the Oncology Business Unit, which is responsible for Keytruda, which is looking beyond its 10th anniversary to a new decade. In the short term, the goal is to expand the indications for which it is being reimbursed, as well as newly approved or pending indications such as endometrial cancer and stomach cancer. “The primary goal is to grow the newly approved indications and to do well until the launch of new indications because there are still indications waiting to be approved,” said Shin. ”The most important thing is to improve patient access to treatment, and we will always think about and work on how to provide good treatment options to more patients.” Next, Shin cited the birth of new drugs that will bring about another paradigm shift as the most anticipated part of the coming decade. “MSD is investing heavily in clinical trials in Korea, which is a global leader in clinical trials, with more than 5,000 people in 500 centers in Korea alone. I believe that another innovative drug will arise as a result of this investment and effort, and in that sense, I think it would be good to look forward to the company’s next 10 years.”
- Policy
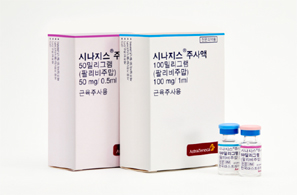
- Shortage of Synagis supply announced…normalized by May
- by Lee, Hye-Kyung Mar 13, 2025 05:57am
- With a shortage in the supply of Synagis (palivizumab), a preventive antibody for respiratory syncytial virus (RSV), which is a main cause of pneumonia and bronchiolitis in infants and young children, expected in Korea, concern arises on how the lack of supply may hinder disease prevention for children in high-risk groups. On the 11th, AstraZeneca Korea notified the Ministry of Food and Drug Safety of a shortage of Synagis 50 mg and 100 mg through a report on supply discontinuation and shortage of drugs. RSV is the most common cause of lower respiratory tract infections in infants and young children, and infants born prematurely, underweight, or with a history of congenital heart disease or bronchopulmonary dysplasia are classified as high-risk groups for RSV infection. Synagis can be administered monthly for 5 months from October to March, during the RSV epidemics season. The first dose should be administered in September before the RSV season begins and should be administered once a month during the RSV season, which lasts until March. The recommended dose is 15 mg per kg of body weight, and the product is available in vials (injections) of 50 mg and 100 mg. AstraZeneca said, “The number of RVS patients has increased more than in previous years, causing a surge in demand for Synagis 50 and 100 mg. Its supply may be temporarily insufficient, due to the current domestic inventory and planned import amounts.” As the hospitalization rate is decreasing as in previous years this March, the current will be of no major concern for patients who have completed receiving the five doses during this RSV epidemic. However, due to the supply shortage in March and April, there is a concern that the preventive effect may be reduced and increase the number of hospitalizations due to RSV infection for the patient group who were unable to receive all the recommended 5 doses. In particular, there is a high possibility that the drug will not be sufficiently effective in preventing the disease in indicated high-risk children. AstraZeneca said, “We will focus on normalizing supply by coordinating the import and domestic release schedules as soon as possible. The expected date for normalization of supply is May 28, 2025.” Meanwhile, Synagis and Sanofi Pasteur Korea's ‘Beyfortus (nirsevimab)' is available in Korea as a preventive vaccine for RSV, but it has different indications. Synagis was approved for the prevention of severe lower respiratory tract disease requiring hospitalization due to RSV in children at high risk of RSV disease, and Beyfortus is indicated for the prevention of lower respiratory tract disease due to RSV. In the case of Synagis, the drug is being administered to high-risk infants and toddlers who are expected to be at high risk of severe RSV disease, such as premature infants.
- Company
- Emergence of a next-generation lung cancer targeted drug?
- by Son, Hyung Min Mar 12, 2025 05:57am
- Clinical trials on 4th generation non-small-cell lung cancer targeted therapies by domestic and international pharmaceutical and biopharmaceutical companies are progressing smoothly. J INTS BIO, Voronoi, Therapex, and BridgeBio are continuing their development making clinical success. Among global pharmaceutical companies, Blue Diamond has entered Phase II clinical trials, and it has been confirmed that Boehringer Ingelheim has shown efficacy with its candidate in preclinical trials. Korean companies make research results studying next generation lung cancer targeted therapies According to industry sources on the 12th, J INTS BIO recently announced the results of a Phase I, high-dose clinical trial of JIN-A02, which is being developed as a treatment for epidermal growth factor receptor (EGFR) positive non-small-cell lung cancer (NSCLC). JIN-A02, a fourth-generation EGFR tyrosine kinase inhibitor (TKI), has a mechanism of action that selectively binds to the C797S mutation that causes resistance to third-generation treatments for non-small cell lung cancer. In clinical trials, high doses (300 mg) of JIN-A02 did not cause any serious adverse reactions or dose-limiting toxicity. To date, JIN-A02 has shown partial response (PR) in 1 patient and stable disease (SD) in 3 patients in clinical trials. J INTS BIO explained that this is the first case of a PR in a patient with the C797S mutation among the fourth-generation EGFR-TKI treatments currently being developed in Korea and abroad. Currently, the first-generation EGFR-positive lung cancer drugs on the market include AstraZeneca's Iressa (gefitinib) and Roche's Tarceva (erlotinib), the second-generation drugs include Boehringer Ingelheim's Giotrif and Pfizer's Vizimpro (dacomitinib), and third-generation drugs, Yuhan Corp’s Leclaza (lasertinib) and AstraZeneca's Tagrisso (osimertinib). However, resistance inevitably develops even with the use of effective targeted therapies. A typical mutation that occurs with EGFR-positive targeted therapies is C797S. In addition, patients lack treatment options to use after targeted therapies. Platinum-based chemotherapy, docetaxel, and immune checkpoint inhibitors are available for patients who develop resistance to targeted therapies. Still, there has been no significant improvement in their response rates with their subsequent use. To address the need, latecomers such as J INTS BIO, have set a goal of confirming the possibility of commercialization by targeting the C797S mutation that occurs after patients develop resistance to existing 1st- to 3rd-generation targeted therapies. Voronoi will present the early clinical results of VRN11 at the American Association for Cancer Research (AACR) Annual Meeting 2025, which will be held next month. According to Voronoi, VRN11 is effective not only against EGFR C797S acquired resistance mutations, but also against common mutations such as EGFR Del19 and L858R, and atypical mutations such as EGFR G719X, L861Q, and S768I. Voronoi recently changed the clinical protocol for VRN11. The company has received approval from the Ministry of Food and Drug Safety to change the clinical trial protocol expand the size of the Phase I clinical trial from 50 to 103 patients and significantly increase the dose escalation rate and target. BridgeBio is exploring the safety and efficacy of BBT-207, which is being developed as a 4th-generation EGFR-positive non-small cell lung cancer treatment, in a Phase I dose-escalation trial. At the recent meeting of the Safety Monitoring Committee, the efficacy and safety of the drug were evaluated by analyzing data from 6 patients enrolled in the fifth dose group of the BBT-207 Phase 1 clinical trial. In the above, BBT-207 did not cause any serious adverse drug reactions, and 3 cases of partial response (PR) and several stable disease (SD) cases were observed. Therapex recently announced the clinical design and interim results of the first cohort of ‘TRX-221,’ a 4th-generation EGFR-targeted anticancer drug for non-small cell lung cancer. TRX-221 is a 4th-generation EGFR tyrosine kinase inhibitor that selectively inhibits EGFR C797S as well as EGFR activating mutations and T790M mutations. Currently, Therapex is conducting a Phase Ia clinical trial on TRX-221 at 6 university hospitals in Korea, including Asan Medical Center, Severance Hospital, and Samsung Medical Center. The company has been administering the drug to patients in the second cohort since early September. Therapex plans to conduct global clinical trials, including in the United States, to expand patient recruitment during the dose development phase. Black Diamond makes the most progress in development... Boehringer also shows its candidate’s potential in preclinical trials Black Diamond Therapeutics has made the most progress in the global pharmaceutical industry. Black Diamond Therapeutics has observed the most PRs with its 4th generation EGFR TKI candidate in the Phase I/II trial. Black Diamond Therapeutics is developing BDTX-1535, which had been developed as a treatment for brain tumors, as a 4th generation lung cancer targeted therapy. According to the results of the Phase II clinical trial that have been disclosed so far, 8 out of 19 patients (42%) treated with the 200 mg dose of BDTX-1535 showed an objective response rate (ORR). Five of the responding patients showed a confirmed partial response (PR), one of whom converted from a PR to an unconfirmed complete response (CR) at the 8-month time point. The safety assessment showed that the 200 mg dose was well tolerated, consistent with previous clinical results. The majority of adverse events were mild or moderate, with rash (70%) and diarrhea (35%) being the most common adverse reactions. There were two cases of Grade 3 rashes, but no Grade 4 rash or Grade 3/4 diarrhea were observed. In particular, Black Diamond explained that its candidate showed promising therapeutic effects in patients who developed resistance after treatment with Tagrisso. Black Diamond plans to update the Phase II clinical trial data within the second quarter of this year. Boehringer Ingelheim is developing BI-4732 as a 4th generation-targeted therapy. It is currently in the preclinical stage. Domestic researchers, including Byoung Chul Cho, director of the Lung Cancer Center at Yonsei Cancer Center, are participating in this clinical trial. In clinical trials that have been disclosed so far, BI-4732 has recorded a cancer cell growth inhibition rate of up to 183% in animal models transplanted with cell lines derived from patients with the triple mutations of exon 19 deletion, T790M, and C797S. This was up to 2.6 times higher than that of Tagrisso. The development of 4th generation lung cancer-targeted therapies is also underway in China. Chinese companies Betta Pharmaceuticals and Chia Tai Tianqing are conducting Phase I clinical trials of BPI-361175 and TQB-3804, respectively.
- Company
- New ADC for breast cancer 'Trodelvy' at the final reimb step
- by Eo, Yun-Ho Mar 12, 2025 05:57am
- A new antibody-drug conjugate (ADC) for breast cancer, 'Trodelvy,' will enter the last stage toward being added to the insurance reimbursement list. The Ministry of Health and Welfare (MOHW) ordered the National Health Insurance Service (NHIS) to begin drug pricing negotiations for Gilead Sciences Korea's triple-negative breast cancer (TNBC) treatment Trodelvy (sacituzumab govitecan). Consequently, negotiations are expected to begin this month (March). Trodelvy has already been listed in about 30 countries worldwide. Since last year, Taiwan has offered health insurance coverage for Trodelvy, which has a national health system like South Korea. Many countries are focusing on quickly improving patient access to Trodelvy due to poor treatment availability for mTNBC and the clinical value of Trodelvy. TNBC is an aggressive form among breast cancer types that is more likely to recur and metastasize. Patients with TNBC who have progressed metastasis despite undergoing chemotherapy have a life expectancy of several months. However, chemotherapy has been used as the standard therapy for a long time because an effective method to target cancer cells has not been discovered. Trodelvy is the first Trop-2 targeted ADC and is the only treatment for mTNBC that is used as second-line treatment or above with a demonstrated survival extension effect compared to chemotherapy. Since its introduction, it has become the standard therapy globally. The current guidelines in the United States and Europe recommend Trodelvy as a priority treatment for patients with mTNBC who have a treatment history. According to the Phase 3 study, patients treated with Trodelvy had an overall survival of 11.8 months, which is close to a year, whereas patients undergoing chemotherapy had 6.9 months. Furthermore, Trodelvy has been shown to be effective in regulating symptoms and pains associated with cancer and improving overall well-being, thereby improving patients' quality of life. Trodelvy received the highest score of 5 on the ESMO-MCBS, a value-evaluation tool for anticancer medicines rated by the European Society for Medical Oncology (ESMO). Drugs with Score of 5 are indicated to not only extend patient survival but also be effective in improving quality of life. Trodelvy is the only drug to receive a Score of 5 among TNBC treatments. Meanwhile, the clinical effectiveness of Trodelvy was demonstrated through the Phase 3 ASCENT study. It has shown a 49% reduction in the risk of death and a 57% improvement in progression-free survival (PFS) in adult patients with unresectable locally advanced or mTNBC who have received two or more prior systemic therapies, including at least one for metastatic disease, compared to patients who received single-agent chemotherapy (TPC, Treatment of Physician’s Choice). These effects were observed regardless of the presence of brain metastasis.
- Policy
- New types of risk-sharing agreements added for reimbursement
- by Lee, Tak-Sun Mar 12, 2025 05:57am
- The Health Insurance Review and Assessment Service has established two types of risk-sharing schemes, including initial treatment cost reimbursement (Fixed cost refund at initial treatment) and outcome-based reimbursement, to the detailed criteria for drugs subject to negotiation. This appears to reflect the additional content included in the recent revision of the Ministry of Health and Welfare's 'Criteria for the Determination and Adjustment of Drugs.’ According to the industry sources on the 7th, the Health Insurance Review and Assessment Service established two types of risk-sharing agreements in the 'Detailed Evaluation Standards for Medicines subject to Negotiations, Including New Drugs'. The fixed cost refund at the initial treatment type, which was added this time, is a contract in which the applicant refunds a certain percentage of the cost of the first dose used for each patient to the National Health Insurance Service. In addition, an outcome-based refund is a type of contract in which the applicant refunds a certain percentage of the total amount billed to the National Health Insurance Service if the treatment effect is not achieved after tracking and observing the treatment effect of each patient for a certain period of time. As a result, the types of risk-sharing schemes have increased from 4 to 6, including conditional treatment continuation type, mixed-refund type, expenditure cap type, refund type, and patient-specific usage cap type. The post-management entities for the additional types have also been set. The National Health Insurance Service will be responsible for post-management of the initial treatment cost reimbursement type, and the Health Insurance Review and Assessment Service will be responsible for post-management of the outcome-based reimbursement type. The newly established types were approved by the Drug Reimbursement Evaluation Committee a meeting held in February. Meanwhile, the 2 RSA types were added to the recently revised Ministry of Health and Welfare's “Criteria for the Determination and Adjustment of Drugs.” The National Health Insurance Service eased the criteria last year to omit cost-effectiveness evaluation for drugs under the risk-sharing scheme with additional claims of less than KRW 1.5 billion. In addition, the ICER threshold flexibility assessment requirement for innovative drugs was newly established, and the first beneficiary item was the breast cancer drug ‘Trodelvy’ in February. In addition, in December, the NHIS decided to simplify the evaluation for RSA drugs subject to a third round of evaluations, along with a series of measures to ease the industry burden.
- Company
- 12 Korean biosimilars globally approved in 3 months
- by Chon, Seung-Hyun Mar 12, 2025 05:56am
- Domestic pharma and biotech companies have achieved 12 biosimilar approvals in the U.S. and Europe this year. Celltrion and Samsung Bioepis have surpassed last year's record of 11 approvals in three months due to their rapid global expansion. Together, Celltrion and Samsung Bioepis have received over 20 biosimilar approvals in Europe and the United States. Celltrion received the U.S. Food and Drug Administration (FDA) approval for Xolair’s biosimilar Omlyclo on the. 10, according to industry sources. Xolair is an antibody biologic drug used to treat allergic asthma, chronic rhinosinusitis with nasal polyps, and chronic idiopathic urticaria. Xolair generated global sales of approximately KRW 6 trillion last year. Omlyclo is the first Xolair biosimilar to be approved in the U.S. and was also the first to be approved in other major global markets including the European Commission (EC), South Korea, the United Kingdom, and Canada. Celltrion has now received approval for a total of 4 biosimilars in the U.S. this year. In January, Celltrion received FDA approval for Avtozma, a biosimilar version of the autoimmune disease treatment Actemra. Actemra is indicated for rheumatoid arthritis (RA), giant cell arteritis (GCA), systemic juvenile idiopathic arthritis (sJIA), polyarticular juvenile idiopathic arthritis (pJIA), and COVID-19. On April 4, Celltrion received FDA approval for the biosimilars Stoboclo and Osenvelt, which are biosimilar versions of Prolia and Xgeva. Prolia and Xgeva are biologics developed by Amgen that differ in the dose and dosing schedule of denosumab. Prolia is approved for the treatment of osteoporosis and Xgeva is approved for the prevention of skeletal-related events in patients with bone metastases and the treatment of giant cell tumors of bone. Number of biosimilars approved by domestic pharmaceutical and biotechnology companies in Europe and the United States by year (Unit: number of items, Source: each company, Financial Supervisory Service) Celltrion has also received 4 biosimilar approvals in Europe this year. Last month, Celltrion received biosimilar approvals for 4 original drugs: Eylea, Actemra, Prolia, and Xgeva. Eylea is indicated for the treatment of ophthalmic conditions including wet macular degeneration, retinal vein occlusion macular edema, and diabetic macular edema. Celltrion received a recommendation for approval of the 4 biosimilars from the European Medicines Agency's Committee for Medicinal Products for Human Use (CHMP) in December of last year and cleared the final approval hurdle within 2 months. This brings the total number of biosimilar approvals Celltrion has received in the U.S. and Europe in the first 3 months of this year to 8, the most ever. This more than doubles the 3 approvals it has received in 2018 and last year in just 3 months. Samsung Bioepis has received a total of 4 biosimilar approvals in the U.S. and Europe this year. Last month, Samsung Bioepis received approvals for 2 biosimilar products Prolia and Xgeva in the U.S. and Europe, respectively. The Prolia biosimilar was approved under the brand name Ospomyv in the U.S. and Obodence in Europe. The Xgeva biosimilar was approved under the brand name Xbryk in both the U.S. and Europe. Celltrion and Samsung Bioepis have received approvals for a total of 12 biosimilars in the U.S. and Europe this year. This surpassed the previous record of 11 approved in a year last year in just 3 months. Last year, 4 domestic pharma and biotech firms achieved 11 biosimilar approvals in the U.S. and Europe. Samsung Bioepis won 3 and 2 biosimilar approvals in the U.S. and Europe, respectively, last year. Samsung Bioepis received U.S. approval for its Eylea biosimilar Opuviz in May last year. In June and July last year, the company received FDA approval for its Stelara biosimilar Pyzchiva and Soliris biosimilar Epysqli, respectively. Samsung Bioepis' Stelara biosimilar Pyzchiva and Eylea biosimilar Opuviz passed through the European gateway in April and November last year. Celltrion received a total of 3 biosimilar approvals in the U.S. and Europe last year. Celltrion's biosimilar to Stelara received FDA approval last year, and biosimilars of Xolair and Stelara reached the commercialization stage in Europe. Dong-A ST won back-to-back FDA approvals in the U.S. and Europe for its biosimilar Imuldosa last year. Korean biopharmaceutical company Prestige Biopharma obtained marketing authorization for its Herceptin biosimilar Tuznue in Europe in September last year. In 2019, Celltrion and Samsung Bioepis received a total of 5 biosimilar approvals in the U.S. and Europe. In November 2019, Celltrion received approval in Europe for Remsima SC, a subcutaneous formulation of Remicade. Remsima SC is a subcutaneous (SC) version of the original intravenous (IV) formulation of Celltrion's proprietary biological drug, Remsima. In 2019, Samsung Bioepis received FDA approval for biosimilars of four products: Herceptin, Enbrel, Humira, and Lucentis. In January 2019, the company received U.S. marketing approval for Herceptin biosimilar Ontruzant, followed by Ethicobo and Hadlima in April and July. The original versions of Ethicobo and Hadlima are Enbrel and Humira, respectively. In September 2021, Samsung Bioepis received U.S. approval for Lucentis' biosimilar Byooviz. In August 2013, Celltrion's Remsima was approved for sale in Europe as the world's first antibody biosimilar, marking the beginning of domestic biosimilars' push into the global market. Since 2016, domestic pharma and biotech companies have continued to achieve new biosimilar approvals in the U.S. or Europe every year. Celltrion and Samsung Bioepis received 24 and 21 approvals in the U.S. and Europe, respectively. Celltrion received 12 and 12 approvals in Europe and the U.S., respectively. Samsung Bioepis' biosimilars received 11 approvals in Europe and 10 in the US.
- Opinion
- [Reporter’s View] Worth of ease in administration
- by Eo, Yun-Ho Mar 11, 2025 05:54am
- You can now orally take injectables, change the daily doses to monthly doses, and manage your disease with once a year injections. Such 'convenience of administration' has now become a competitive edge in the pharmaceutical market. Convenience, which had been mainly emphasized for chronic diseases, is now being highlighted in various other disease areas including anticancer drugs and autoimmune diseases. The emergence of one-shot drugs played a role, but other advanced new drugs have also been putting forth not only their efficacy but also their convenience. The convenience of administration literally means 'that taking the medication is convenient.' The question arises, 'If I'm taking medication because I'm sick, is comfort really that important? Shouldn't a drug’s efficacy be the most important aspect for the company?’ Nevertheless, pharmaceutical companies are quite obsessed with showing off their drug’s convenience. In many cases, convenience is the main slogan for marketing and sales of the relevant drugs. As such, “convenience” is emerging as a keyword in the healthcare industry. However, the value of such ‘convenience’ is not very recognized in the process of reimbursement listing. In particular, health authorities tend to be reluctant to accept the higher price of next-generation drugs that offer improved convenience compared to existing drugs for severe diseases such as cancer, which is a life-threatening disease. From the government’s standpoint, it’s a valid argument. If a drug receives a higher price simply because of its improved convenience, this may rather lower the opportunity cost for patients with other diseases that share the nation’s limited budget. However, there is a high possibility of new drugs that offer improved convenience while showing the same efficacy as existing drugs being introduced to the market. However, convenience is not unconditionally important. It depends on the situation. Common sense dictates that in the case of cancer, which is a life-threatening condition, there are not many cases where the prescription is changed for the sake of convenience. Therefore, the convenience of such anticancer drugs must be accompanied by a significant breakthrough in the method of administration or offer improved efficacy. A doctor would not give a patient a new drug if he or she is seeing efficacy with their currently prescribed drug, as this may cause unexpected side effects. In addition, the convenience of the drug may be reduced when it is used as part of a combination therapy regimen or when the patient has relevant diseases. However, it is also worth considering whether blindly rejecting the worth of convenience in administration is right. There are cases where such improvements in convenience can lead to improvements in treatment outcomes. There are also cases where long-acting drugs reduce the burden of monitoring and save health finances. In the current healthcare trend where cancer is also turning into a chronic disease, the 'quality of life' of the patients cannot be ignored. Convenience is a value that is difficult to either blindly support or ignore. However, if a drug has been developed to treat a major condition, that values convenience in administration, the value of this drug that met this need should be recognized, amid the increasing number of drugs that are facing difficulties in the listing process that offer improved convenience.