- LOGIN
- MemberShip
- 2026-01-19 21:05:31
- GLP-1’s expansion has only just begun
- by Son, Hyung Min | translator | 2026-01-16 08:52:26

SGLT-2 inhibitors, which began as treatments for type 2 diabetes, have established themselves as standard-of-care (SOC) after demonstrating clinical efficacy in major metabolic diseases such as heart failure and chronic kidney disease.
Beyond blood glucose control, they have demonstrated cardiovascular and renal protective effects and the potential to improve long-term prognosis. As a result, they are regarded as having reshaped the treatment paradigm itself, beyond being drugs for specific diseases.
Now, GLP-1–based therapies are taking over that baton.
GLP-1 drugs have expanded their indications from type 2 diabetes to obesity, cardiovascular disease, kidney disease, and metabolic dysfunction–associated steatohepatitis (MASH), evolving into comprehensive treatment options covering the full spectrum of metabolic disease. Recently, their potential has even been discussed in Alzheimer’s disease, addiction disorders, and cancer prevention, raising the possibility that GLP-1 drugs could evolve into a systemic therapeutic platform rather than a single-disease therapy.
This shift is most clearly evident in Novo Nordisk's ‘Ozempic/Wegovy (semaglutide)’ and Eli Lilly's ‘Mounjaro (tirzepatide)’, the leading GLP-1 drugs in the global market. While both drugs originated in diabetes, they have since differentiated themselves through distinct strategies for expanding indications and selecting mechanisms, broadening their respective domains.
Building on the clinical and commercial success of GLP-1 drugs, domestic and multinational pharmaceutical companies are accelerating efforts to simultaneously pursue indication expansion and next-generation mechanism development, significantly broadening the scope of the GLP-1 market. The industry is now watching closely whether GLP-1 drugs can move beyond their single category as obesity treatments to become the core pillar for metabolic diseases overall.
Beyond obesity and diabetes to cardiovascular, renal, and liver diseases... the current state of GLP-1 expansion
The expansion of indications for GLP-1 receptor agonists is already translating into actual approvals and late-stage clinical results.
Originally developed for obesity and type 2 diabetes, GLP-1 drugs are now extending into cardiovascular disease, kidney disease, and MASH, positioning themselves as treatment options covering the entire spectrum of metabolic disorders.
Notably, semaglutide expanded its therapeutic scope beyond obesity and diabetes treatments by securing evidence for reducing major adverse cardiovascular events (MACE).
By accumulating clinical evidence encompassing not only diabetes patients but also high-risk cardiovascular populations, GLP-1 therapies have clearly demonstrated their potential to contribute to long-term prognosis improvement beyond weight loss.
In addition, it has expanded into chronic kidney disease (CKD), strengthening its presence in a field once pioneered by SGLT-2 inhibitors.
More recently, accelerated approval for MASH has pushed GLP-1 drugs into liver disease territory.
Their mechanistic strengths in terms of weight loss, improved insulin resistance, and anti-inflammatory effects have translated into reduced hepatic fat accumulation and improved fibrosis, making them a promising new option in an area with limited therapeutic choices.
Tirzepatide is also rapidly expanding its footprint. Beyond obesity and diabetes, it has secured approval for obstructive sleep apnea (OSA) and continues clinical development in cardiovascular and renal disease.
Its strategy centers on managing obesity-related complications simultaneously, leveraging its strong weight-loss efficacy.
This demonstrates how GLP-1 agonists are evolving beyond merely treating obesity-associated conditions to become therapies capable of modulating the pathophysiology of the disease itself.
From single-mechanism drugs to multi-agonists … evolves to a ‘platform technology’
If indication expansion represents the competition over what can be treated, diversification of mechanisms and delivery methods represents competition over how to treat. The GLP-1 market has already moved beyond single-mechanism competition and entered the development phase for multi-agonist combination therapies that target multiple receptors simultaneously.
Early GLP-1 drugs focused on appetite suppression and satiety enhancement. However, dual and triple receptor agonists that simultaneously modulate various hormone receptors involved in metabolic regulation, such as glucagon-like peptide-1 (GLP-1), glucagon (GCG), and amylin receptor agonists, have recently emerged in succession.
This multi-agonist approach differentiates itself by maximizing weight loss effects while also targeting lipid metabolism improvement, increased energy expenditure, and long-term metabolic stability.
Dual GLP-1/GIP agonists, represented by Mounjaro, simultaneously promote insulin secretion and improve insulin resistance, while triple GLP-1/GIP/GCG agonists are evaluated as a strategy to further enhance weight loss and metabolic improvement effects.
With the addition of GLP-1/amylin combination strategies, the GLP-1 class itself is effectively being redefined not as a single drug class but as a therapeutic platform. Recently, Novo Nordisk completed clinical trials for its platform therapy ‘CagriSema (semaglutide/caglinotide)’ and submitted a marketing application to the U.S. Food and Drug Administration (FDA). The mechanism aims for additional effects, such as delayed glucose absorption in the intestine, by combining semaglutide with caglinotide.
Competition over delivery methods is also underway. While most currently commercialized obesity treatments are injectables, new delivery options like long-acting injections, oral formulations, and patches have entered the clinical stage.
Oral GLP-1 agents, in particular, are seen as a key variable that could significantly expand treatment accessibility by reducing the psychological burden associated with injections. This holds the potential to broaden the target population for obesity treatment from specialized care settings to routine chronic disease management.
Novo Nordisk and Lilly are leading in this area. Novo Nordisk has already secured approval for its oral Wegovy formulation, while Lilly has completed clinical trials for ‘Oroforglifron’ and submitted its FDA application. Unlike Mounjaro, Oroforglifron utilizes a single GLP-1 receptor agonist.
Long-acting injectables are also rapidly entering late-stage clinical trials. Amgen's ‘MariTide’ uses an amino acid linker to conjugate two GLP-1 molecules with a fully human monoclonal anti-human GIPR antibody. This is known to produce greater weight loss effects than GLP-1 monotherapy.
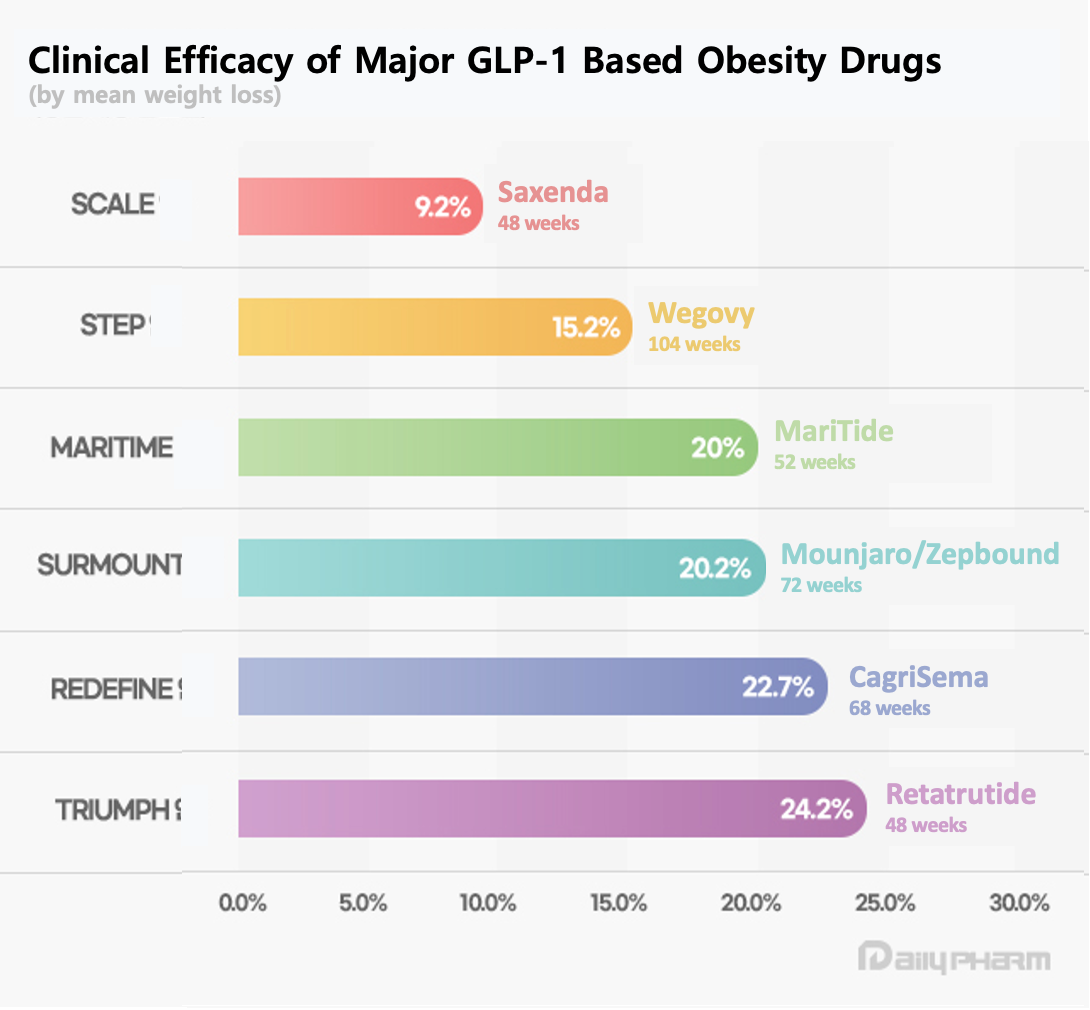
Since existing GLP-1 obesity treatments like Saxenda, Wegovy, and Zepbound became global blockbuster drugs, the pharmaceutical industry has been racing to develop new formulations. The existing drug Saxenda requires once-daily administration, while Wegovy and Zepbound require weekly injections. This is why a long-acting injectable formulation is expected to gain a competitive edge in terms of treatment convenience if commercialized.
In Phase II clinical trials, MariTide demonstrated a maximum 20% weight loss rate at week 52 in non-diabetic obese patients. Based on this data, Amgen has fully launched the global Phase III clinical study, MARITIME. Amgen also plans to initiate Phase III studies this year for atherosclerotic cardiovascular disease (ASCVD), heart failure (HF), and obstructive sleep apnea (OSA).
Domestic and multinational pharmaceutical companies are actively joining this trend. Multinational pharmaceutical firms are pursuing strategies to preempt next-generation standard treatments through multi-mechanism pipelines, while domestic pharmaceutical and biotech companies are also joining the global competition by leveraging dual/triple receptor agonists and novel delivery technologies.
Ultimately, the competition in GLP-1 mechanisms and delivery methods is converging toward simultaneously fulfilling efficacy, convenience, and long-term manageability. This suggests that GLP-1 therapies are establishing themselves not as a temporary trend, but as a core platform for chronic metabolic disease treatment that will endure for decades to come.
Latecomers are conducting clinical trials focused on differentiating themselves from existing drugs in areas like administration convenience, quality of weight loss, and preventing rebound effects. Next-generation obesity drugs that enable greater weight loss with a single dose are analyzed to potentially surpass existing drugs in administration convenience. With indications poised to expand, the value of obesity drugs is also being set against the benchmark of an average weight loss rate of 20%.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.






