- LOGIN
- MemberShip
- 2025-12-22 08:07:52
- Hanmi-MSD collaborate for R&D
- by Cha, Jihyun | translator Hong, Ji Yeon | 2025-05-20 05:59:10
Hanmi Pharmaceutical entered into a clinical trial collaboration agreement with the U.S.-based Merck (MSD) for developing an immune anticancer drug candidate.
The clinical collaboration between Hanmi Pharmaceutical (hereafter, Hanmi) and Merck has expanded to three cases.
In addition to clinical trial collaboration, Hanmi continues to collaborate with MSD for efforts such as technology transfers.
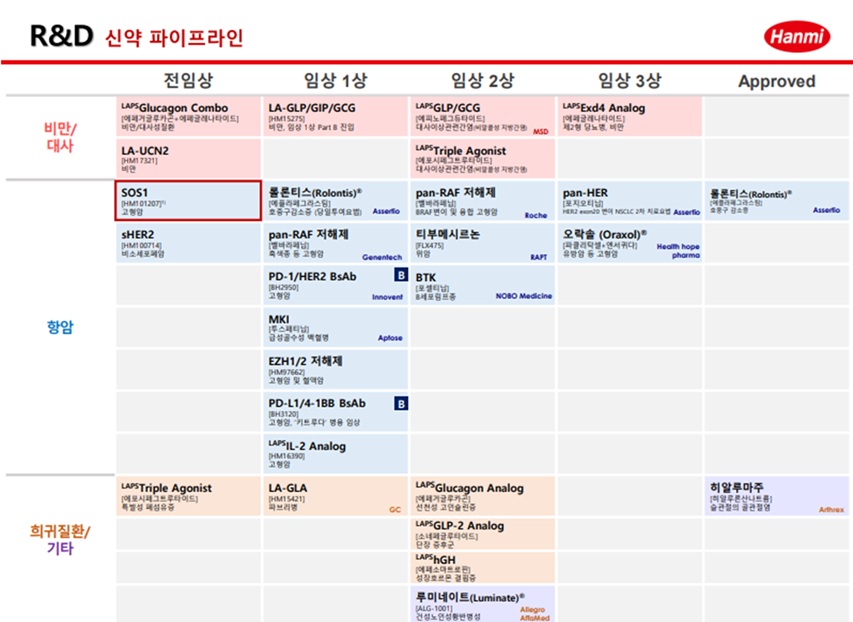
According to pharmaceutical sources on May 20, Hanmi recently signed a clinical collaboration and distribution agreement with MSD to evaluate the combination therapy containing 'HM16390,' a next-generation anticancer drug that is "LAPS interleukin-2 (IL-2) analog," and MSD's anti-PD-1 anticancer drug 'Keytruda (active ingredient name: pembrolizumab).
According to the agreement, Hanmi will be responsible for conducting the Phase 1 trial to assess the safety and efficacy of HM16390+Keytruda combination therapy as a clinical trial sponsor.
MSD will supply Keytruda used in clinical trials.
HM16390 is a next-generation interleukin-based immuno-oncology candidate that activates immune cells.
HM16390 induces T-cell proliferation and activation to enhance immune responses within the tumor microenvironment.
By increasing the number of tumor-infiltrating lymphocytes (TIL) that respond to immune checkpoint inhibitors in the tumor microenvironment, it is designed to convert 'cold tumors' (with low immune activity) into 'hot tumors' (infiltrated by immune cells) and to maximize efficacy when used in combination with checkpoint inhibitors.
'Proleukin' is the only recombinant IL-2 therapy approved by the U.S.
Food and Drug Administration (FDA).
Still, its use is limited due to the risk of adverse events at high doses.
Most IL-2 analogs in development focus on enhancing binding affinity to the IL-2 β-receptor to boost anti-tumor effects, which can trigger excessive systemic immune responses and lead to severe side effects such as cytokine release syndrome.
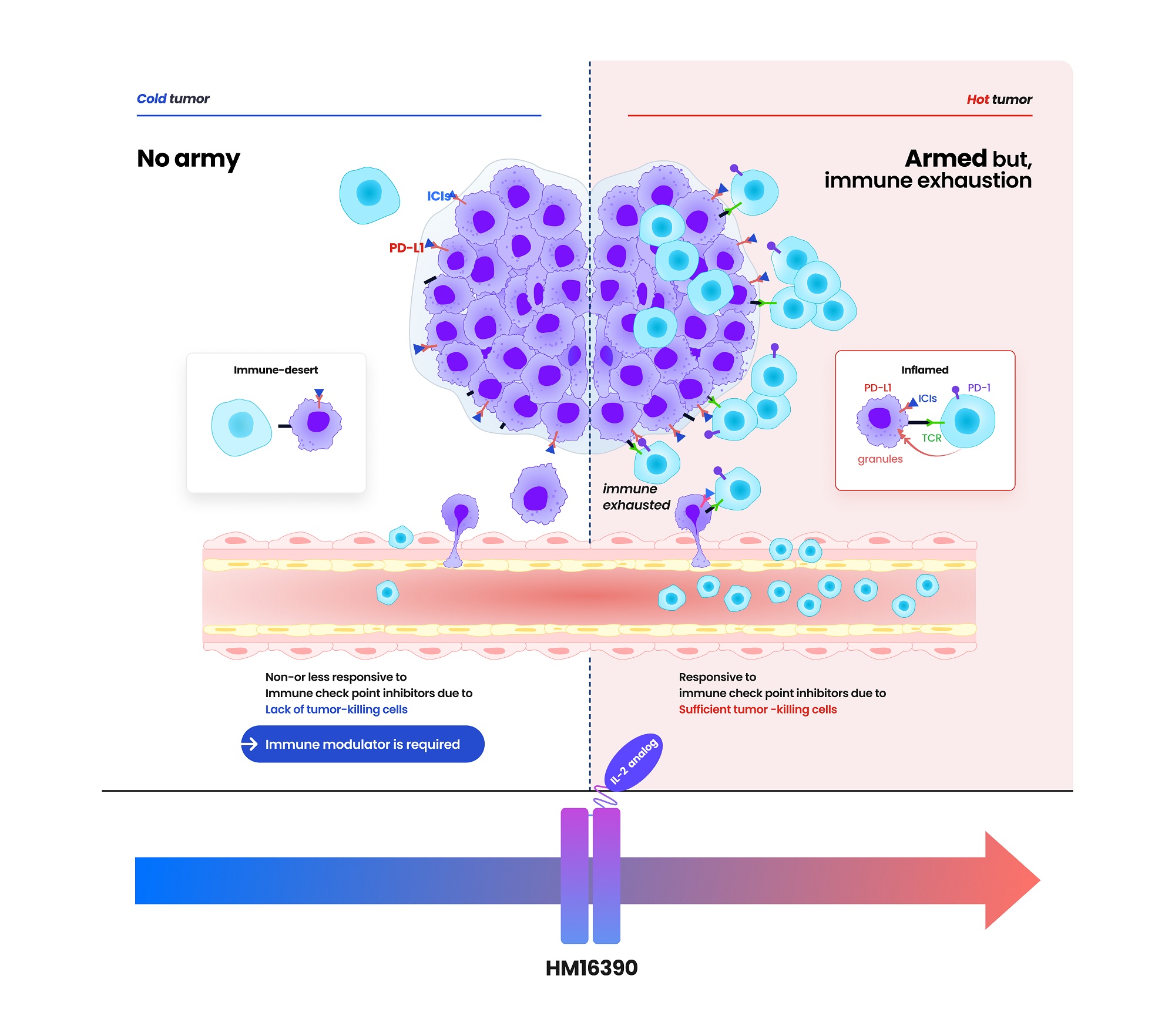
According to Hanmi, this approach secures safety while maximizing efficacy.
In particular, Hanmi is developing HM16390 as a long-acting therapeutic using its proprietary Labsccovery platform technology, enabling once-per-cycle subcutaneous (SC) administration alongside chemotherapy.
HM16390 is currently in a multinational Phase 1 clinical trial.
Earlier, Hanmi announced at the Society for Immunotherapy of Cancer (SITC) last November that preclinical studies of HM16390 demonstrated complete remission.
Keytruda, MSD's immuno-oncology agent, is the world's top-selling pharmaceutical.
It is an immune checkpoint inhibitor that blocks the interaction between PD-1 on T cells and PD-L1 on cancer cells, enabling immune cells to attack tumor cells.
First approved by the U.S.
FDA in September 2014 for malignant melanoma, Keytruda has continuously added new indications.
It has over 40 indications to date, including breast, gastric, and lung cancers, making it the checkpoint inhibitor with the broadest range of cancer uses.
However, the efficacy of Keytruda is limited to patients whose tumors express high levels of PD-L1.
It is known that those with low PD-L1 expression derive minimal benefit.
To address this, MSD is actively developing combination therapies to expand the responsive patient population and enhance efficacy.
Hanmi expects that combining HM16390 with Keytruda will further improve treatment outcomes.
Hanmi and MSD have expanded collaboration deals to three in total.
Hanmi also conducts Keytruda combination therapy clinical trials with MSD's PD-L1/4-1BB bispecific antibody candidate 'BH3120' in combination with its oral CCR4 antagonist, 'Tivumecirnon.' BH3120, which is being co-developed by Hanmi and Beijing Hanmi Pharm, is a bispecific antibody immuno-oncology candidate in which a single antibody simultaneously binds to two different targets.
It features Hanmi's proprietary Pentambody platform technology, combining an immuno-oncology mechanism that activates immune cells with the target-oncology characteristic of selectively attacking only cancer cells.
In particular, Hanmi explains that BH3120 is designed to respond differently to each target, PD-L1 and 4-1BB, thereby enhancing therapeutic efficacy while reducing side effects.
Hanmi plans to present the interim results of the Phase 1 clinical trial evaluating the BH3120 and Keytruda combination therapy in the second half of this year.
Previously, Hanmi Pharmaceutical reported that in tumor-bearing mouse models refractory to immune-oncology agents, the combination of BH3120 and Keytruda demonstrated tumor growth inhibition that was at least comparable to that observed with a competing pipeline agent (GEN1046).
It blocks the CCR4 receptor protein, thereby inhibiting the migration of regulatory T cells that suppress immune responses into tumors.
In January, at the ASCO Gastrointestinal Cancers Symposium held in San Francisco, Hanmi presented a poster on the Phase 2, Part 1 results for Tivumecirnon.
In collaboration with RAPT and MSD, this trial treated ten Epstein-Barr virus (EBV)-positive gastric cancer patients and achieved an objective response rate (ORR) of 60%.
ORR is a key efficacy metric representing the proportion of patients whose tumors have either disappeared entirely or shrunk by a defined amount following cancer treatment.
Among these responses, there was one complete response and five partial responses.
The median time to response (mTTR) was 2.7 months.
The median duration of response (mDOR) was 17.3 months.
In Cohort 2, the median progression-free survival (PFS) was 10.4 months.
Hanmi explained that the treatment-related adverse events observed among the 20 patients enrolled in the trial were mostly manageable.
In addition to its clinical collaboration with MSD, which is focused on Keytruda combination therapy, Hanmi is also maintaining partnerships via technology licensing agreements.
For instance, it includes 'efpeglenatide,' which Hanmi licensed to MSD in 2020 in a deal valued at USD 860 million.
Efpeglenatide is a dual-action agent that activates the glucagon-like peptide-1 (GLP-1) receptor, which enhances insulin secretion and suppresses appetite, and the glucagon receptor, which increases energy metabolism.
Hanmi previously licensed efpeglenatid to Janssen in 2015 for obesity and diabetes indications, regained the rights in 2019, repurposed it for metabolic-associated steatohepatitis (MASH), and successfully licensed it again to MSD.
MSD is currently conducting a Phase 2 clinical trial of efpeglenatide.
The trial compares efpeglenatide with the comparator treatment, semaglutide from Novo Nordisk, and a placebo.
According to a 2023 presentation at the European Association for the Study of the Liver (EASL) in Vienna, data from the Phase 2a analysis showed that at week 24 of treatment, efpeglenatide reduced liver stiffness by 72.7% compared to baseline.
This result markedly outperformed semaglutide, which achieved a 42.3% reduction over the same period.
Resolution of steatosis without fibrosis worsening and improvement of fibrosis without steatosis worsening are key evaluation endpoints defined by the FDA for NASH therapies.
MSD aims to complete the Phase 2 trial of efpeglenatide by December of this year.
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.










