JAKi Cibinqo owns strengths in dosage adjustments
... switching should be reimbursed
Jung, Sae-Im 기자 2023-05-15 12:03:04
Pfizer’s JAK inhibitor to likely receive reimbursement for atopic dermatitis within the first half of the year
Dose can be adjusted according to treatment progress... frequency of acne side effects low
Shows effect after switching to Cibinqo depending on whether a patient shows an effect with Dupixent...”can serve as for reimbursement extensions”
Pfizer's JAK inhibitor ‘Cibinqo (abrocitinib)' has embarked on a full-fledged journey to expand its prescriptions. After landing in major general hospitals at the end of last year, the drug is likely to be registered for reimbursement within the first half of this year.
Cibinqo is a Janus kinase 1 (JAK1) inhibitor approved by the Ministry of Food and Drug Safety in November 2021. It is the 4th JAK inhibitor introduced to Korea and the second JAK inhibitor drug introduced by Pfizer after Xeljanz. Unlike Xeljanz, which is only used for ulcerative colitis, Cibinqo is used to treat severe atopic dermatitis.
Treatment options have increased significantly for severe atopic dermatitis starting with the introduction of the injection-type biologic medication and oral JAI inhibitors. In particular, a total of 3 oral JAK inhibitors are available for patient use in Korea. Olumiant (baricitinib) and Rinvoq (upadacitinib) have first entered the market and are being used with reimbursement, followed by Cibinqo.
Although Cibinqo is a latecomer, it owns a differentia table property from existing drugs. Dailypharm met with Jung-Im Na, Professor of Dermatology at Seoul National University Bundang Hospital to hear about its differentiable properties in the field.
▲ Jung-Im Na, Professor of Dermatology at Seoul National University Bundang Hospital
Its first advantage is in its free dose control. Cibinqo comes in three doses: 50, 100, and 200mg. Although the recommended starting dose is 200mg, the dosage can be adjusted to 100 mg or 50 mg depending on the progress of treatment. If symptoms worsen with dose reduction, the doctor can increase treatment response again by increasing the dose and using local treatment together (JADE REGIMEN study).
Professor Na said, “JAK inhibitors are fast and effective, but its exit strategy is considered a problem after the patient’s condition improves. In other words, ending treatment after seeing an effect is difficult with the use of JAK inhibitors. However still, due to its free dosage adjustments, it is attractive that the dosage can be reduced step by step from 200mg to 50mg.”
He added, “No drug can be used for the rest of one’s life. Therefore, how to complete the treatment well is an important factor, and dose plays an important role. In particular, JAK inhibitors generally have a short half-life, and therefore disappear quickly from the body, leading to recurrence. Therefore, you cannot terminate treatment at once,” emphasizing the importance of dosage control.
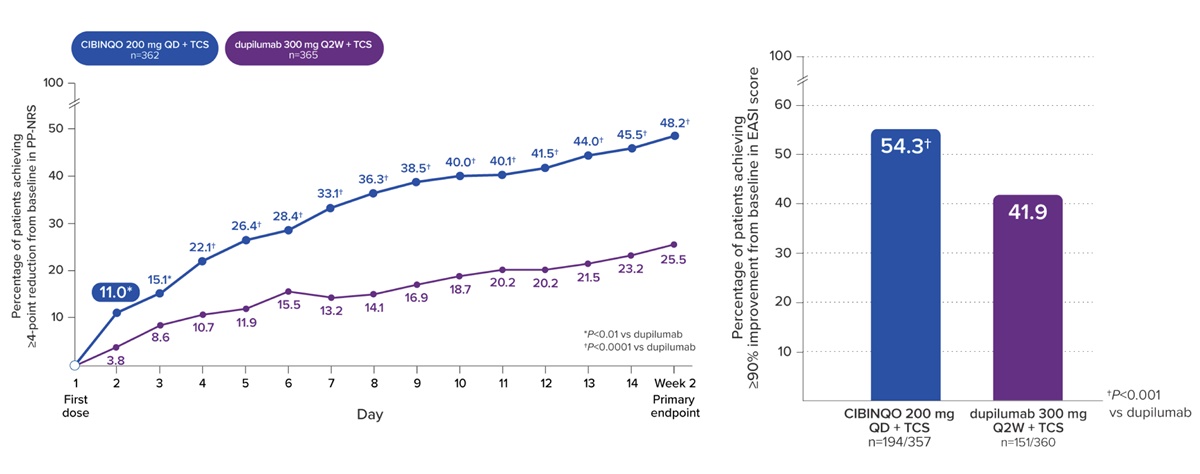
▲ Major efficacy endpoint results in the JADE DARE trial
Another advantage is in how it holds grounds based on a head-to-head trial for switching to the biological drug Dupixent. Among Cibinqo’s 7 Phase III clinical trials, the JADE DARE study is a head-to-head clinical trial comparing Cibinqo with Dupixent. As a result of separately administering Cibinqo 200mg and dupilumab 300mg in combination with a topical treatment for 26 weeks, the proportion of patients who achieved an improvement of 4 points or larger in the Peak Pruritus Numerical Rating Scale (PP-NRS4) at 2 weeks was 48.2% in the Cibinqo group, higher than the 25.5% of the Dupixent group. The proportion of patients who achieved a 90% improvement in the Eczema Area and Severity Index (EASI-90) at Week 4 was also significantly higher in the Cibinqo group (28.5%) than in the Dupixent group (14.6%).
Moreover, the company also conducted the JADE EXTEND study, which studied the effect of switching patients who used Dupixent to Cibinqo. Patients were divided into those who were responsive to Dupixent and those that were non-responsive to Dupixent. 93.5% and 90.2% of patients who showed a response to Dupixent achieved EASI-75 after switching to Cibinqo 200mg and 100mg, respectively. PP-NRS4 was 89.7% and 81.6%, respectively. Among patients who did not respond to Dupxient, the proportion of those that reached EASI-75 was 80% and 67.7%, respectively, and PP-NRS4 was 77.3% and 37.8%, respectively.
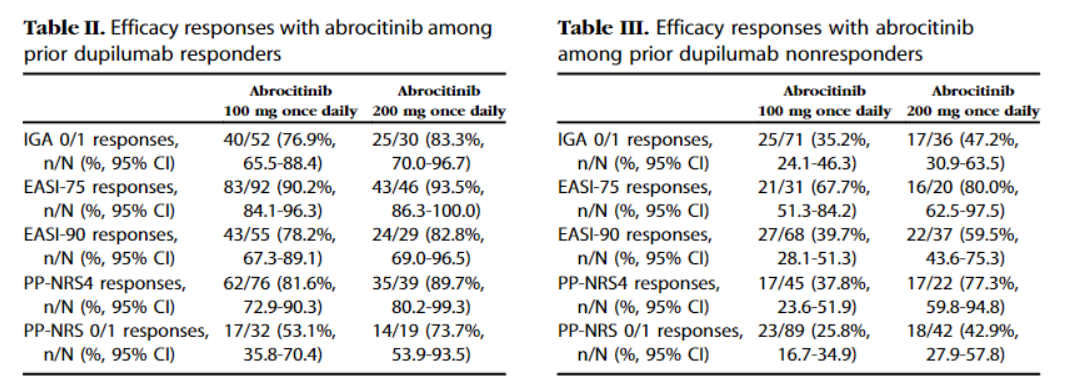
▲ Efficacy of switchin gto Cibinqo(Abrocitinib) among patients thatresponsive to Dupxient(left) and those that were unresponsive (Data: Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab).
The study provided evidence that patients who did not see an effect with Dupixent can expect an effect when they switch to Cibinqo. Previously, a clinical trial was also conducted with the JAK inhibitor Rinvoq on switching therapy from Dupixent. However, Cibinqo is the only drug that analyzed the effect of switching administration according to the patient’s presence or absence of an effect with Dupixent.
This is why the trials have raised expectations of JAK inhibitors receiving expanded reimbursement as a replacement therapy when switching from Dupixent.
Professor Na said, “The reason why the JADE EXTEND clinical trial is significant is that there are many patients who do not see an effect while using Dupixent, even though it is a good drug. However, these patients have to discontinue treatment and use immunotherapies for 3 months and see a deterioration in their condition before switching to a different treatment to receive reimbursement due to limited reimbursement standards. As Cibinqo has evidence prepared with clinical trials, the trials may be grounds to extend the drug’s reimbursement to switching medications. Also, For those who have not seen any effect after using Dupixent, the evidence is there to switch to Civinco.”
Jung, Sae-Im 기자 (same@dailypharm.com)