Biopharmaceutical CDMO annual average of ↑31%
[Bio Korea] 30 domestic CGT majors, need to overcome the size difference
Lee, Hye-Kyung 기자 2023-05-11 05:50:52
10 billion dollars in 2026
Resolving the risk of outsourcing genes by using an automated system

▲ Kwon Soon-jae, managing director of ENCell, is giving a presentation on the current status of the CDMO market at Bio Korea 2023.
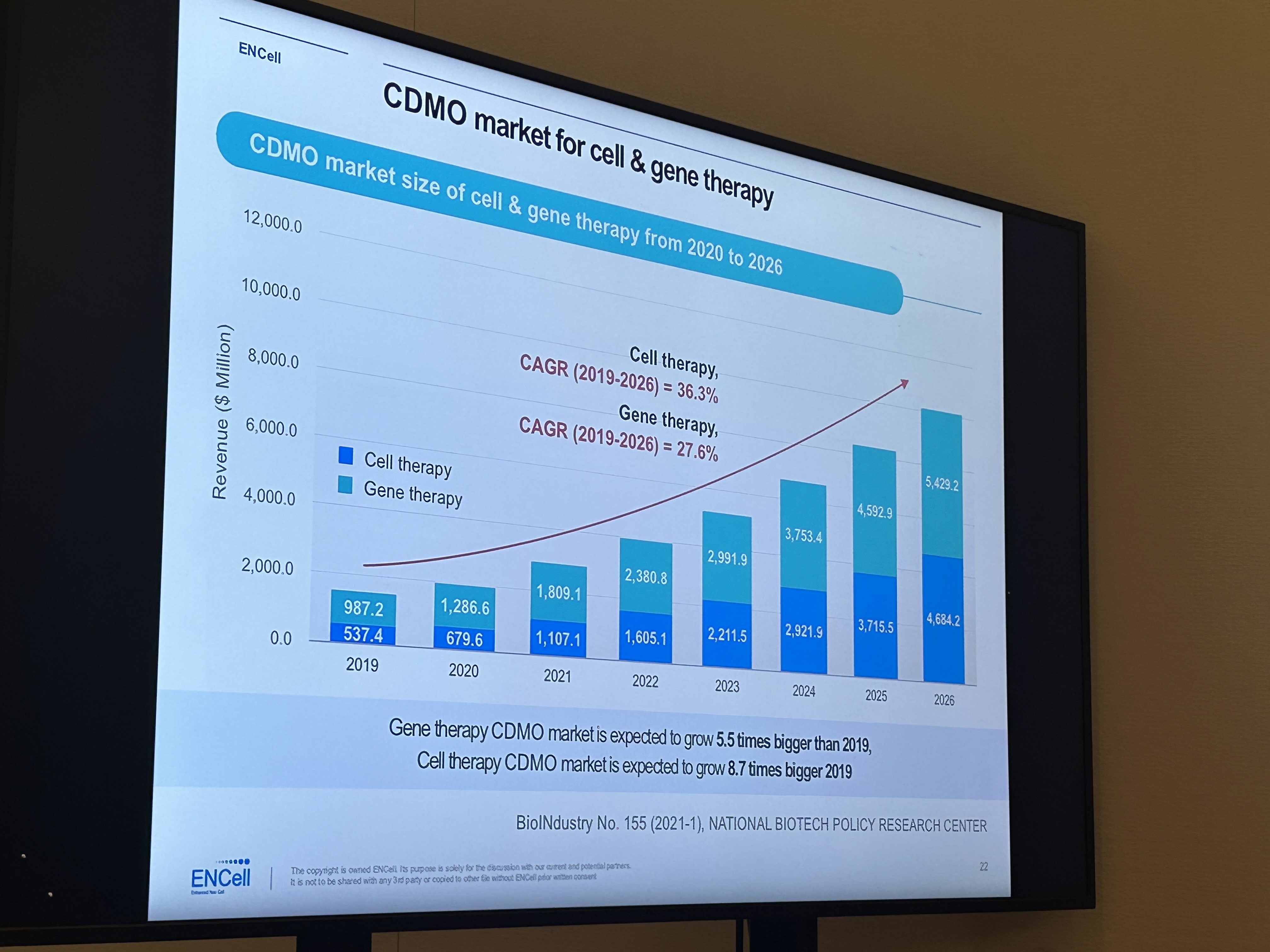
Director Kwon explained, "Cell & Gene Therapy is expected to grow 5.5 times in 2026 compared to 2019, and gene therapy is expected to grow 8.7 times." He explained, "If you look at the high CAGR from 2019 to 2026, it will account for 36.3% and 27.6%, respectively." The demand for CMOs and CDMOs has increased due to the COVID-19 pandemic, and Director Kwon said, "Small companies use CMOs and CDMOs to reduce costs and time, while large companies use CMOs and CDMOs to reduce marketing and R&D costs." It plays a part," he said.
However, in the case of domestic CGT treatment, the manufacturing technology is complicated and the number of platforms is not large, so it was inevitable to create a GMP facility with an 'in-house' concept rather than CDMO service, and have many in-house processes and services. Director Kwon said, "However, as the requirements of the Ministry of Food and Drug Safety become stricter, infrastructure, raw materials, facility costs, labor costs rise, and technology becomes more complex, outsourcing instead of in-house is becoming a trend." Looking at the domestic CGT CDMO market, Lonza, Samsung Bio, SK, CJ, Lotte, and Medipost have announced their entry into the CMO/CDMO business following Thermoficer in 2017.
Director Kwon said, "Most of the 30 CGT companies in Korea are major companies, and 80% of them are trying to develop AAC, adenovirus, CAR-T, etc., and only 30% of them are investing more than 2 million dollars." "If you look at the CGT market alone, it's still the first step, the introductory stage," he said. Director Kwon said that the present, when the first step of CDMO in the CGT market was taken, is an important point in determining the future. Director Kwon said, "More than 100 companies worldwide have entered the CDMO cell gene therapy market, and price, location, and regulations are challenges to be resolved." It looks like I'll have to give it a try," he said.
Lee, Hye-Kyung 기자 (hgrace7@dailypharm.com)