SK Biopharm’s cenobamate secures KRW 700 billion
Power of the new FDA-approved drug
Chon, Seung-Hyun 기자 2023-05-11 12:04:05
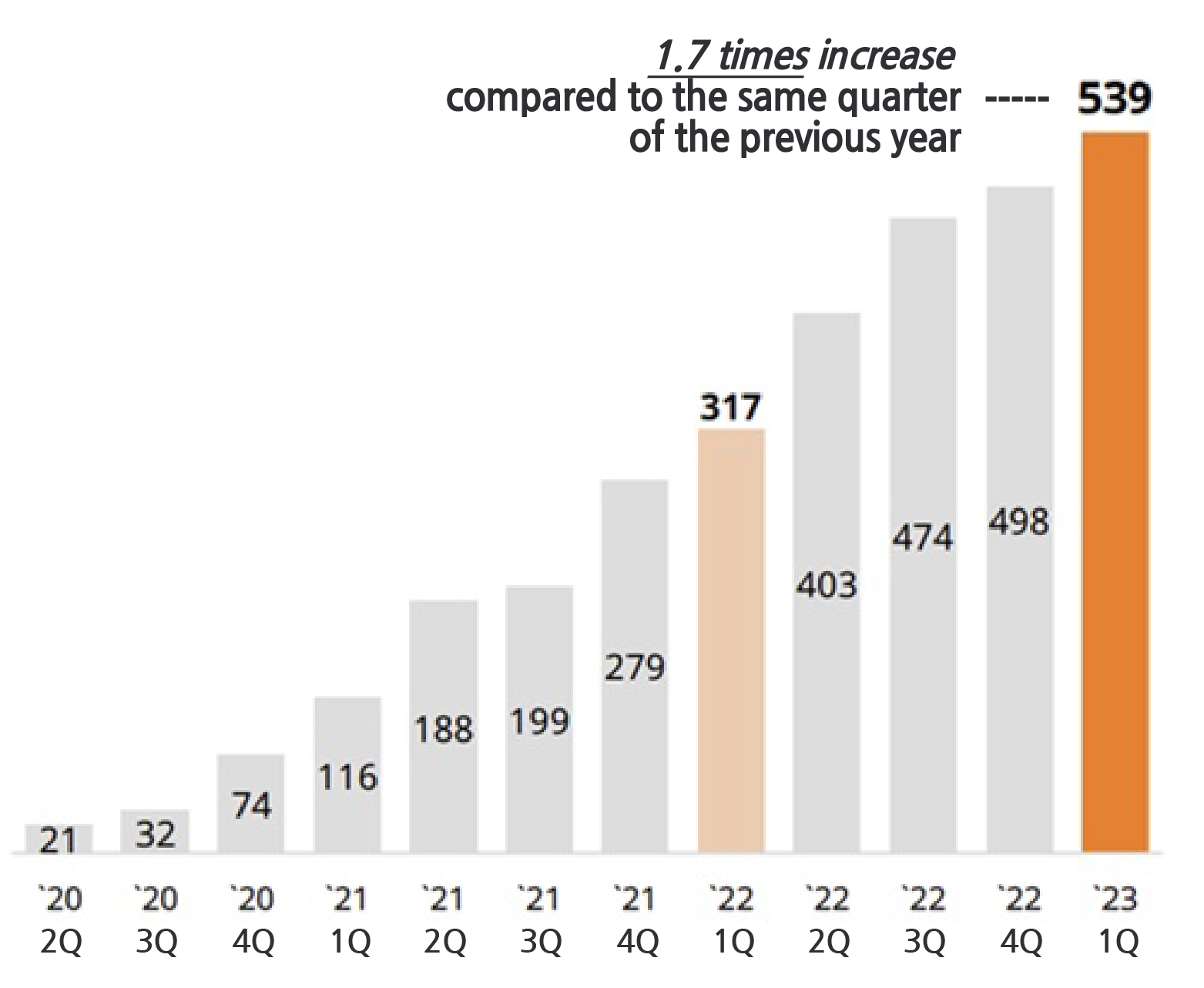
Q1 sales of cenobamate recorded KRW 53.9 billion...shows growth every quarter since its release
Cumulative sales in the US record KRW 314 billion...brought in over KRW 400 billion as technology fees since 2019
Cumulative sales of SK Biopharmaceutical’s epilepsy treatment cenobamate exceeded KRW 300 billion in the US. It had continued to show growth every quarter ever since its release. Combined with the upfront payment and milestone payments cenobamate has earned more than KRW 700 billion over the past 4 years.
According to SK Biopharm on the 11th, cenobamate posted sales of KRW 53.9 billion in the United States in Q1 last year. This is a 70.0% increase from the KRW 31.7 billion made in the same period last year. This also surpassed the previous record of KRW 49.8 billion recorded in the previous quarter by 8.2%, and the sales showed continued growth every quarter since its release.
The total number of prescriptions for cenobamate in the United States is also on the rise. The total number of prescriptions in the first quarter was about 55,000, up 10% from the previous quarter.
Cenobamate is a new anti-epileptic developed solely by SK Biopharmaceuticals from its initial development to US FDA approval as a treatment for partial-onset seizures in adults. It simultaneously regulates 2 targets related to excitatory/inhibitory signaling that are known to cause epilepsy to reduce seizure frequency.
SK Biopharmaceuticals received approval for cenobamate under the brand name ‘Xcopri’ from the US FDA in November 2019, and has been directly selling the drug through its US subsidiary, SK Life Science since May 2020.
Cenobamate has been growing every quarter since generating initial sales of KRW 2.1 billion in Q2 2020. In Q1 2021, sales exceeded KRW 10 billion, and quarterly sales exceeded KRW 50 billion this year. Cumulative sales of cenobamate in the US totaled KRW 314 billion.
Also, Cenobamate has secured over KRW 400 billion as technology fees over the past 4 years.
SK Biopharmaceuticals entered into an exclusive licensing agreement in February 2019 with the Swiss pharmaceutical company Arvelle Therapeutics to transfer technology on cenobamate for up to USD 530 million. At the time, SK Biopharmaceuticals received an upfront payment of USD 100 million with no obligation of return.
In October 2020, the company entered into an exclusive licensing agreement with Ono Pharmaceutical for Ono to develop and commercialize Xcopri in Japan. Under the agreement, SK Biopharmaceuticals received an upfront payment of ¥5 billion with no obligation of return, and will also be eligible to receive up to ¥48.1 billion based on the achievement of certain regulatory and commercial milestones, as well as over 10% royalties on net sales generated in Japan.
In November 2021, SK Biopharmaceuticals licensed out 6 new central nervous systems (CNS) drugs including cenobamate to Ignis Therapeutics. Under the deal, SK Biopharmaceuticals received an upfront payment of USD 20 million, a milestone payment of USD 15 million, and royalties on net sales in the future. Through the technology export, SK Biopharmaceuticals acquired 150 million shares of Ignis (share amounts to 44.9% including common stock).
And in December 2021, SK Biopharmaceuticals signed a licensing deal with Endo Group for the commercialization of its epilepsy drug cenobamate across Canada. Under the deal, SK Biopharmaceuticals an upfront payment of USD 20 million. The company will also be able to receive up to USD 21 million in Canadian dollars based on the achievement of certain regulatory and commercial milestones in the future. Paladin Labs Inc., a Canada-based operating subsidiary of Endo, will be responsible for all commercial activities related to cenobamate in the region, including its release. Endo is a global healthcare company headquartered in Ireland.
In July last year, SK Biopharmaceuticals signed a licensing out deal with the Brazilian pharmaceutical company Eurofarma Laboratorios SA for cenobamate. Under the agreement, SK Biopharmaceuticals will receive an upfront payment of USD 15 million and up to USD 47 million in milestone payments. Under the licensing out agreement, Eurofarma will be selling cenobamate in 17 Latin American countries including Brazil and Mexico
In addition to upfront payments, the company has also received milestone payments upon cenobamate’s approval abroad.
SK Biopharmaceuticals received USD 123.22 million from its European partner Angelini Pharma as milestone payments last year. Angelini Pharma (formerly Arvelle Therapeutics UK) has collected additional milestone payments after receiving marketing authorization from the European Commission in March last year.
SK Biopharmaceutical’s cash inflow from upfront payments and further milestones from the technology transfer of cenobamate is USD 278.22 million and ¥5 billion. Based on recent exchange rates, the company had secured about KRW 400 billion through upfront and milestone payments through technology transfer with cenobamate. Combined with US sales, the drug had brought in over KRW 700 billion.
The company is seeking to expand its sales in the global market. After its approval in Europe in March 2021, the company released its drug under the product name ‘Ontozry.’ So far, it has been released in 18 European countries, including Germany, England, Italy, Spain, and France.
The company is also speeding up development to expand indications for cenobamate as well as its pipeline. Cenobamate is undergoing multinational clinical trials to extend its indication to generalized seizures and expand the age group that can be administered from adults to adolescents, and the study has entered Phase III trials in Korea.
An SK Biopharmaceuticals official said, “We plan to conduct aggressive sales activities including improving the incentive system for our sales representatives to encourage sales in the US and by expanding the clientele from epilepsy specialists to general neurologists.”
According to SK Biopharm on the 11th, cenobamate posted sales of KRW 53.9 billion in the United States in Q1 last year. This is a 70.0% increase from the KRW 31.7 billion made in the same period last year. This also surpassed the previous record of KRW 49.8 billion recorded in the previous quarter by 8.2%, and the sales showed continued growth every quarter since its release.
The total number of prescriptions for cenobamate in the United States is also on the rise. The total number of prescriptions in the first quarter was about 55,000, up 10% from the previous quarter.

▲ Quarterly sales of cenobamate in the US (Unit: KRW 100 million, Data: SK Biopharm).
Cenobamate is a new anti-epileptic developed solely by SK Biopharmaceuticals from its initial development to US FDA approval as a treatment for partial-onset seizures in adults. It simultaneously regulates 2 targets related to excitatory/inhibitory signaling that are known to cause epilepsy to reduce seizure frequency.
SK Biopharmaceuticals received approval for cenobamate under the brand name ‘Xcopri’ from the US FDA in November 2019, and has been directly selling the drug through its US subsidiary, SK Life Science since May 2020.
Cenobamate has been growing every quarter since generating initial sales of KRW 2.1 billion in Q2 2020. In Q1 2021, sales exceeded KRW 10 billion, and quarterly sales exceeded KRW 50 billion this year. Cumulative sales of cenobamate in the US totaled KRW 314 billion.
Also, Cenobamate has secured over KRW 400 billion as technology fees over the past 4 years.
SK Biopharmaceuticals entered into an exclusive licensing agreement in February 2019 with the Swiss pharmaceutical company Arvelle Therapeutics to transfer technology on cenobamate for up to USD 530 million. At the time, SK Biopharmaceuticals received an upfront payment of USD 100 million with no obligation of return.
In October 2020, the company entered into an exclusive licensing agreement with Ono Pharmaceutical for Ono to develop and commercialize Xcopri in Japan. Under the agreement, SK Biopharmaceuticals received an upfront payment of ¥5 billion with no obligation of return, and will also be eligible to receive up to ¥48.1 billion based on the achievement of certain regulatory and commercial milestones, as well as over 10% royalties on net sales generated in Japan.
In November 2021, SK Biopharmaceuticals licensed out 6 new central nervous systems (CNS) drugs including cenobamate to Ignis Therapeutics. Under the deal, SK Biopharmaceuticals received an upfront payment of USD 20 million, a milestone payment of USD 15 million, and royalties on net sales in the future. Through the technology export, SK Biopharmaceuticals acquired 150 million shares of Ignis (share amounts to 44.9% including common stock).
And in December 2021, SK Biopharmaceuticals signed a licensing deal with Endo Group for the commercialization of its epilepsy drug cenobamate across Canada. Under the deal, SK Biopharmaceuticals an upfront payment of USD 20 million. The company will also be able to receive up to USD 21 million in Canadian dollars based on the achievement of certain regulatory and commercial milestones in the future. Paladin Labs Inc., a Canada-based operating subsidiary of Endo, will be responsible for all commercial activities related to cenobamate in the region, including its release. Endo is a global healthcare company headquartered in Ireland.
In July last year, SK Biopharmaceuticals signed a licensing out deal with the Brazilian pharmaceutical company Eurofarma Laboratorios SA for cenobamate. Under the agreement, SK Biopharmaceuticals will receive an upfront payment of USD 15 million and up to USD 47 million in milestone payments. Under the licensing out agreement, Eurofarma will be selling cenobamate in 17 Latin American countries including Brazil and Mexico
In addition to upfront payments, the company has also received milestone payments upon cenobamate’s approval abroad.
SK Biopharmaceuticals received USD 123.22 million from its European partner Angelini Pharma as milestone payments last year. Angelini Pharma (formerly Arvelle Therapeutics UK) has collected additional milestone payments after receiving marketing authorization from the European Commission in March last year.
SK Biopharmaceutical’s cash inflow from upfront payments and further milestones from the technology transfer of cenobamate is USD 278.22 million and ¥5 billion. Based on recent exchange rates, the company had secured about KRW 400 billion through upfront and milestone payments through technology transfer with cenobamate. Combined with US sales, the drug had brought in over KRW 700 billion.
The company is seeking to expand its sales in the global market. After its approval in Europe in March 2021, the company released its drug under the product name ‘Ontozry.’ So far, it has been released in 18 European countries, including Germany, England, Italy, Spain, and France.
The company is also speeding up development to expand indications for cenobamate as well as its pipeline. Cenobamate is undergoing multinational clinical trials to extend its indication to generalized seizures and expand the age group that can be administered from adults to adolescents, and the study has entered Phase III trials in Korea.
An SK Biopharmaceuticals official said, “We plan to conduct aggressive sales activities including improving the incentive system for our sales representatives to encourage sales in the US and by expanding the clientele from epilepsy specialists to general neurologists.”
Chon, Seung-Hyun 기자 (1000@dailypharm.com)